Etoricoxib tablets and preparation method thereof
A technology for etoricoxib and oxic tablets, applied in the field of etoricoxib tablets and their preparation, can solve the problems of reduced particle solubility and/or stability, reduced drug dissolution, and influence on preparation disintegration aging, and achieves avoiding solubility and stability. Reduced drug properties, optimize disintegration and dissolution properties, and avoid the effects of drug dissolution reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Specifically, the preparation method includes:
[0026] S1. Mix etoricoxib, microcrystalline cellulose, disintegrant and part of lubricant, and dry granulate to obtain granule product.
[0027] Specifically, step S1 includes:
[0028] S1.1 Prepare the raw materials according to the required ratio.
[0029] Among them, in order to facilitate the subsequent preparation and ensure the uniformity of mixing, optionally, the particle size of etoricoxib does not exceed 30 mesh, the particle size of anhydrous calcium hydrogen phosphate does not exceed 30 mesh, and the particle size of disintegrant does not exceed 30 mesh. mesh, and the particle size of the lubricant does not exceed 120 mesh.
[0030] If the particle size of the raw material does not meet the above requirements, the raw material should be sieved first to obtain the undersize material that meets the target particle size, and the undersize material of each raw material should be prepared according to the require...
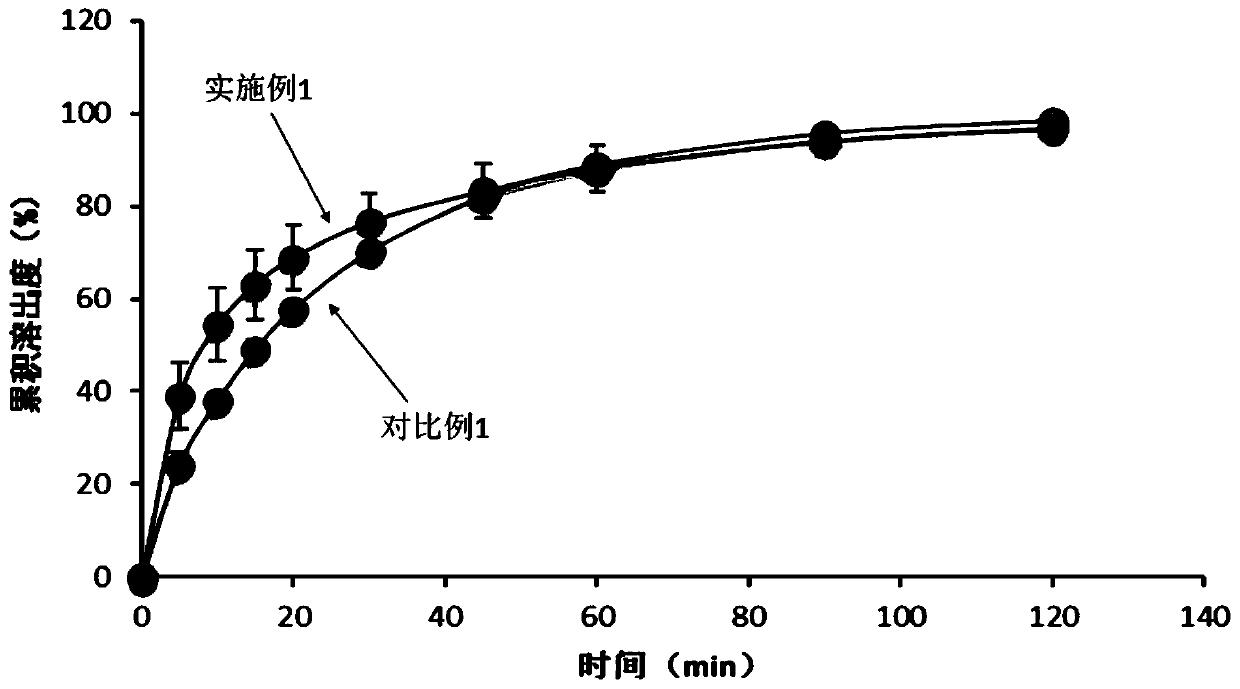
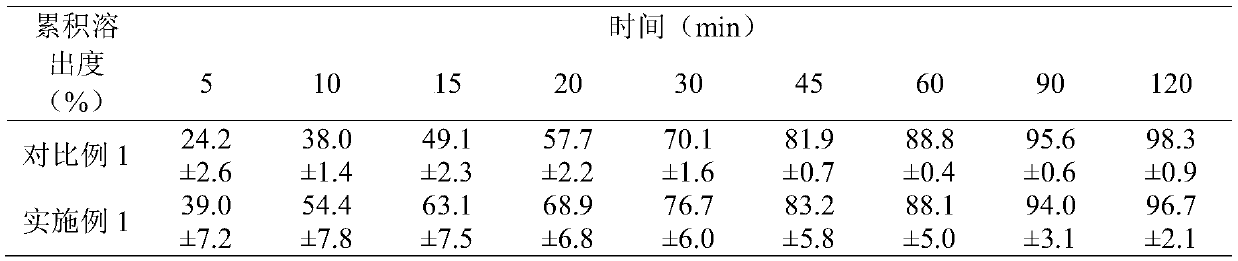
Embodiment 1
[0060] An etoricoxib tablet, in terms of mass percentage, comprising: etoricoxib 24.58%, microcrystalline cellulose 35.24%, anhydrous calcium hydrogen phosphate 28.57%, disintegrant 1.9%, lubricant 0.95%, and coating Materials 8.76%.
[0061] Etoricoxib tablets are prepared by the following methods:
[0062] Mix etoricoxib with a particle size of not more than 30 mesh, microcrystalline cellulose with a particle size of not more than 30 mesh, croscarmellose sodium with a particle size of not more than 30 mesh and some magnesium stearate with a particle size of not more than 120 mesh , dry granulation, granulation, to obtain granular products with a particle size of no more than 20 mesh.
[0063] Mix the granular product, the rest of magnesium stearate and anhydrous calcium hydrogen phosphate with a particle size not exceeding 120 meshes, press into tablets, and coat with a coating material to obtain etoricoxib tablets.
[0064] Wherein, the microcrystalline cellulose is micro...
Embodiment 2
[0066] An etoricoxib tablet, in terms of mass percentage, comprising: etoricoxib 36.50%, microcrystalline cellulose 21.35%, anhydrous calcium hydrogen phosphate 28.99%, disintegrant 3%, lubricant 1.14%, and coating Materials 9.02%.
[0067] The preparation method of etoricoxib tablets is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com