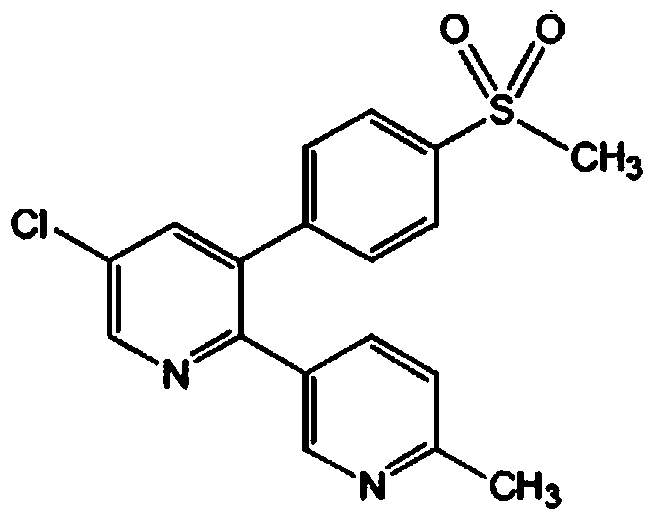
A kind of etoricoxib oral microemulsion preparation and preparation method thereof
A technology of etoricoxib and milk preparations, applied in anti-inflammatory agents, pharmaceutical formulations, emulsion delivery, etc., can solve the problems of poor solubility and low bioavailability, and achieve the goal of masking bitterness, increasing solubility, and improving bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
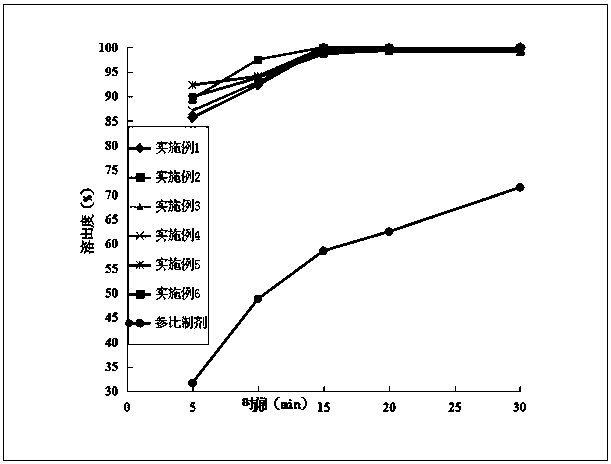
Image
Examples
Embodiment 1
[0034] Prescription composition:
[0035] Name of raw material percentage 10 prescription bottles etoricoxib 1% 1g Soybean oil 3% 3g polyoxyethylene castor oil 5% 5g Propylene Glycol 4% 4g polyethylene glycol 400 3% 3g sodium benzoate 0.1% 0.1g Deionized water 83.9% 83.9g
[0036] Preparation Process:
[0037] (1) Take 1g of etoricoxib, add 3g of soybean oil to dissolve at room temperature;
[0038] (2) Add 5 g of polyoxyethylene castor oil, 4 g of propylene glycol and 3 g of polyethylene glycol 400 into (1), stir magnetically for 5 min, add about 28 g of deionized water, and continue stirring for 10 min to form a coarse milk;
[0039] (3) Transfer the mixture in (2) to a high-pressure homogenizer, add 0.1g of sodium benzoate, add the remaining 55.9g of deionized water, turn on the high-pressure homogenizer, perform high-pressure homogenization for 5 minutes, and repeat 3 times to prepare micro dairy products...
Embodiment 2
[0041] Prescription composition:
[0042] Name of raw material percentage 10 prescription bottles etoricoxib 1% 1g Medium-chain triglycerides 5% 5g ethyl oleate 2% 2g
[0043] polyoxyethylene castor oil 7% 7g glycerin 10% 10g aspartame 0.1% 0.1g lemon zest 0.25% 0.25g sodium benzoate 0.2 0.2g distilled water 74.45% 74.45g
[0044] Preparation Process:
[0045] (1) Take 1g etoricoxib, add 5g medium carbon chain triglycerides and 2g ethyl oleate to dissolve at room temperature;
[0046] (2) Add 7g of polyoxyethylene castor oil and 10g of glycerin to (1), sonicate for 10min, add 25g of distilled water and continue sonicating for 10min to form a coarse milk;
[0047] (3) Transfer the mixture in (2) to a high-pressure homogenizer, add 0.1g of aspartame, 0.25g of lemon essence, add the remaining 49.45g of distilled water, turn on the high-pressure homogenizer, homogenize under high pressure f...
Embodiment 3
[0049] Prescription composition:
[0050] Name of raw material percentage 100 prescription bottles etoricoxib 2% 2g castor oil 10% 10g Polyoxyethylene Hydrogenated Castor Oil 7% 7g Propylene Glycol 10% 10g aspartame 0.2% 0.2g pineapple flavor 0.25% 0.25g sodium benzoate 0.3% 0.3g Deionized water 70.25% 70.25g
[0051] Preparation Process:
[0052] (1) Take 2g of etoricoxib, add it to 10g of castor oil and heat to 40°C to dissolve;
[0053] (2) Add 7 g of polyoxyethylene castor oil and 10 g of propylene glycol into (1), stir mechanically for 10 min, add 23 g of deionized water, and continue stirring for 10 min to form a coarse milk.
[0054](3) Transfer the mixture in (2) to a high-pressure homogenizer, add 0.2g aspartame, 0.25g pineapple essence and 0.3g sodium benzoate, add the remaining 47.25g deionized water, turn on the high-pressure homogenizer, and Homogenize for 5 minutes and repeat 3 times to p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com