Preparation method of etoricoxib crystal form
A technology of etoricoxib and crystal form, applied in the field of preparation of etoricoxib V crystal, can solve the problems of poor stability, low yield, low purity and the like, and achieves lower unit price of raw materials, high yield and good process reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: comparative embodiment, do not add crystal seed crystallization situation
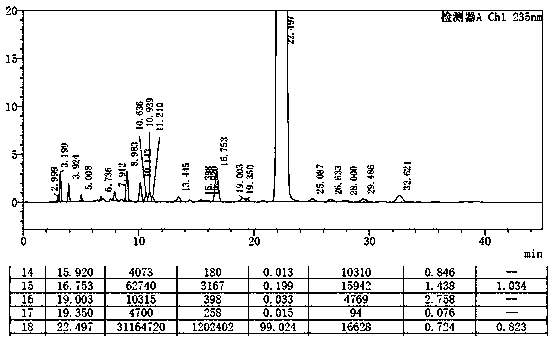
[0030] Add 5g of etoricoxib and 15ml of isopropyl acetate into the three-neck flask, heat to 75°C to dissolve the solid completely, keep stirring at this temperature (75°C) for 1 hour, then slowly cool down to room temperature, stir for 2 hours, and filter. The obtained solid was air-dried at 60°C for 12 hours to obtain 4.04 g of crystal form V etoricoxib with a purity of 99.0%, a yield of 80.8%, and a specific impurity of ET-IMP-409 of 0.199%. See the attachment for the liquid phase determination spectrum of the finished product figure 1 .
Embodiment 2
[0031] Example 2: Preparation of V crystal form etoricoxib
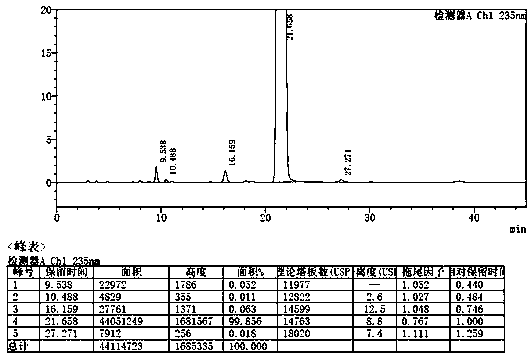
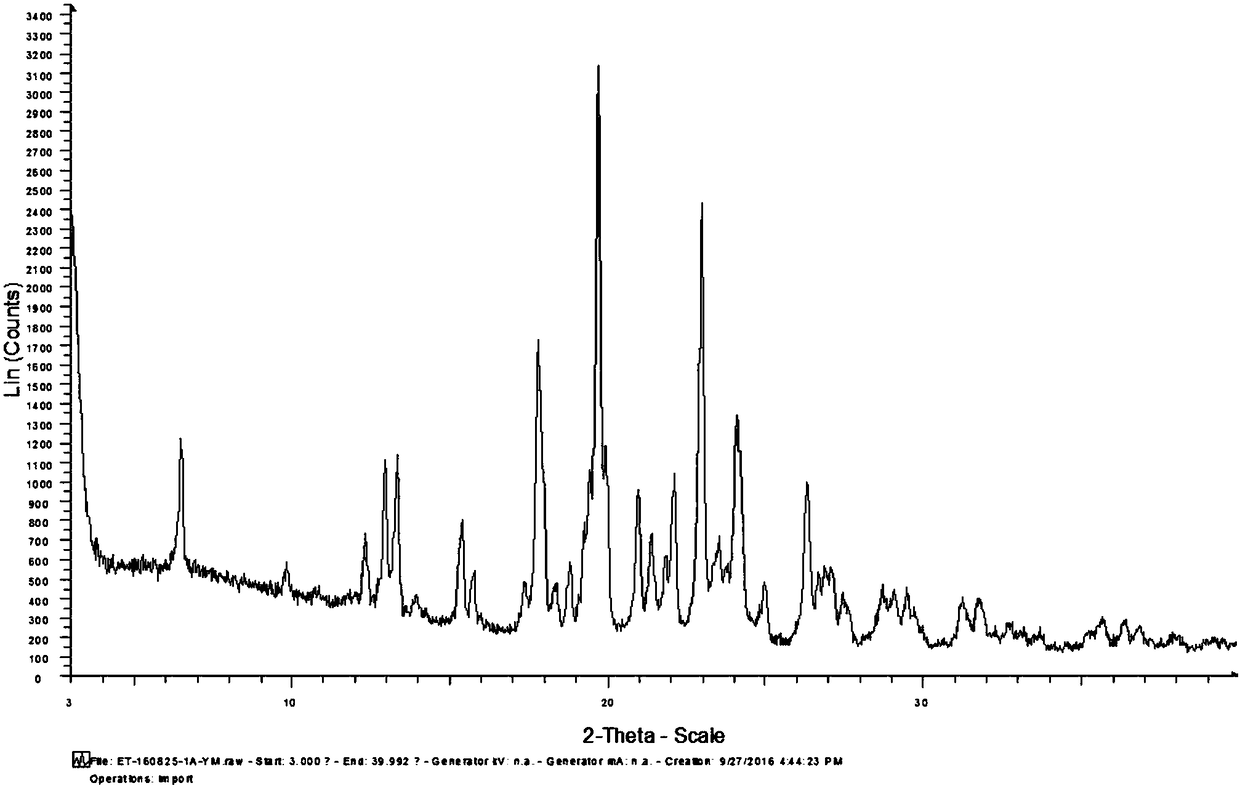
[0032] Add 5g etoricoxib and 15ml isopropyl acetate into the three-necked flask, heat to 75°C to dissolve the solid completely, add 0.25g (5%) etoricoxib crystal form V seed crystal at this temperature (75°C), Insulated and stirred for 1h, then cooled to room temperature at a cooling rate of 10°C / h, stirred for 2h, filtered, and the resulting solid was air-dried at 60°C for 12h to obtain 4.64g of crystal form V etoricoxib with a purity of 99.86% and a yield of 92.8%. The specific impurity ET-IMP-409 is 0.063%, and the liquid phase determination spectrum of the finished product purity is shown in the attachment figure 2 , see the attached crystal form diffraction pattern of the finished product image 3 .
Embodiment 3
[0033] Example 3: Preparation of V crystal form etoricoxib
[0034] Add 5g etoricoxib and 15ml isopropyl acetate into the three-neck flask, heat to 75°C to dissolve the solid completely, add 0.15g (3%) etoricoxib crystal form V seed crystal at this temperature (75°C), Insulated and stirred for 1 hour, then cooled to room temperature at a cooling rate of 20°C / h, stirred for 2 hours, filtered, and the resulting solid was air-dried at 60°C for 12 hours to obtain 4.61 g of crystal form V etoricoxib with a purity of 99.95% and a yield of 92.2%.
[0035] Example 3: Preparation of V crystal form etoricoxib
[0036] Add 5g of etoricoxib and 15ml of isopropyl acetate into the three-neck flask, heat to 75°C to dissolve the solid completely, add 0.15g (3%) etoricoxib crystal form V seed crystal at 72°C, keep stirring for 1h, Afterwards, the temperature was lowered to room temperature at a cooling rate of 5°C / h, stirred for 2h, filtered, and the obtained solid was air-dried at 60°C for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com