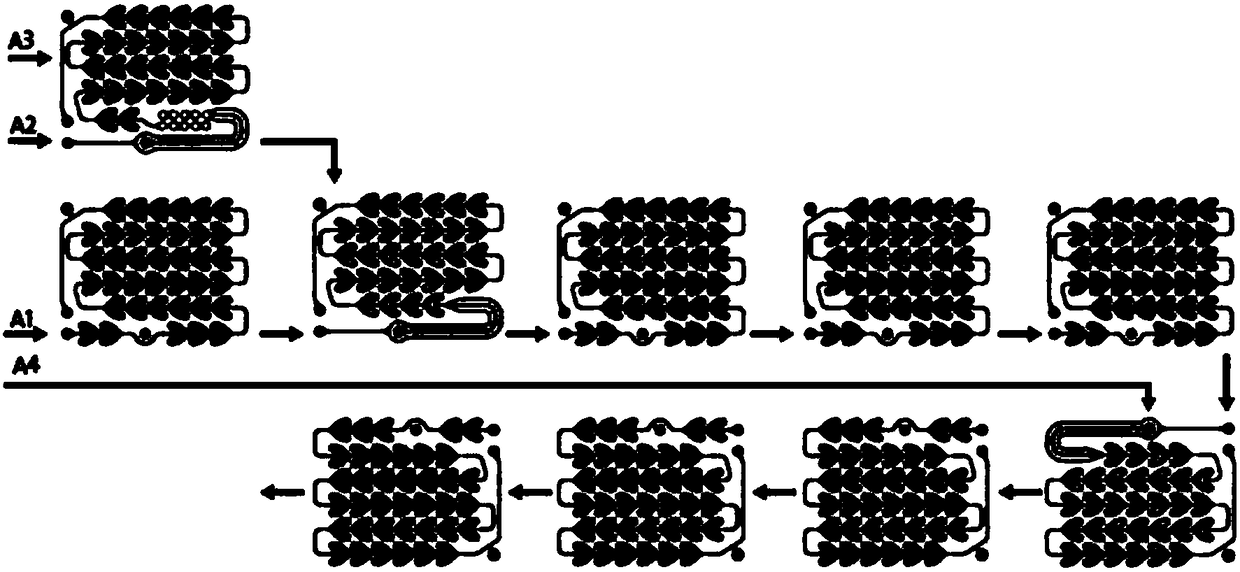
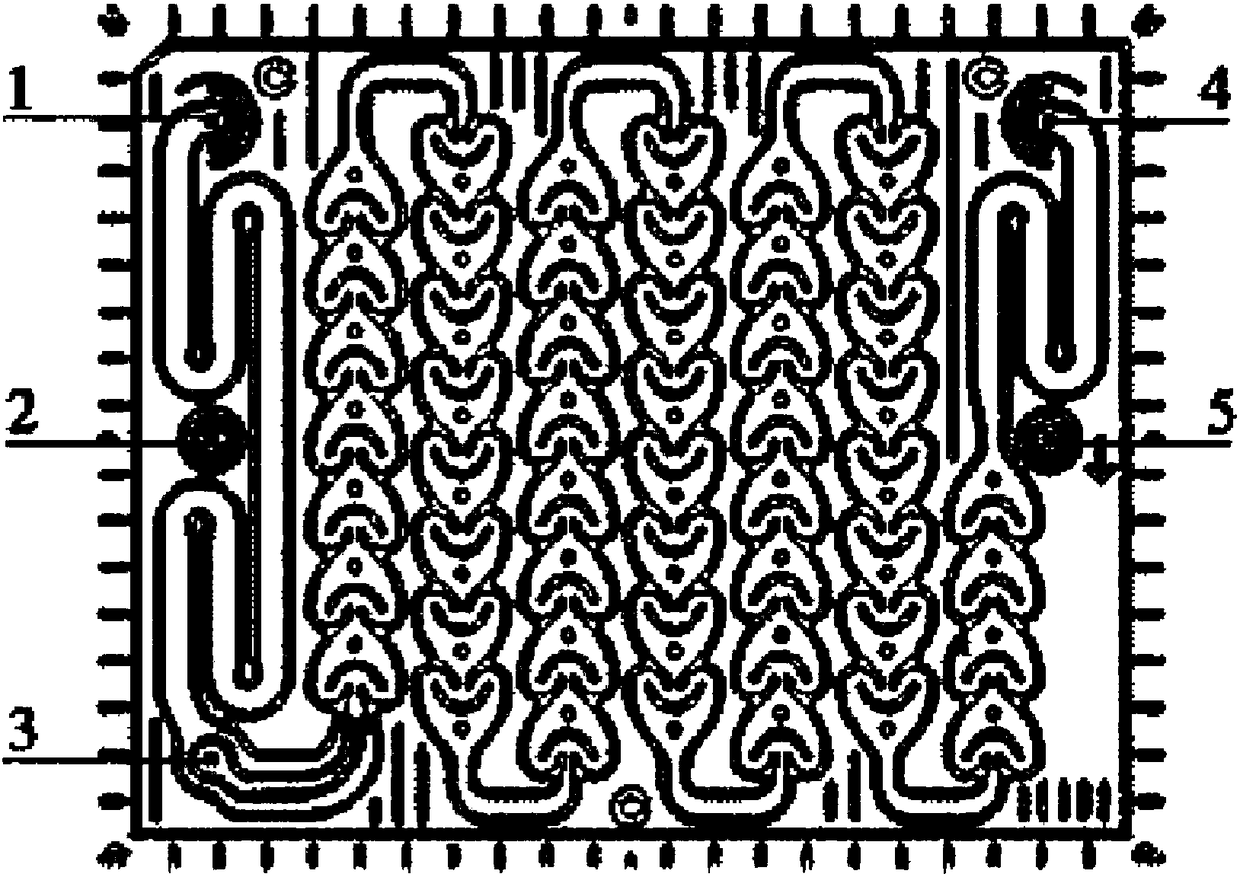
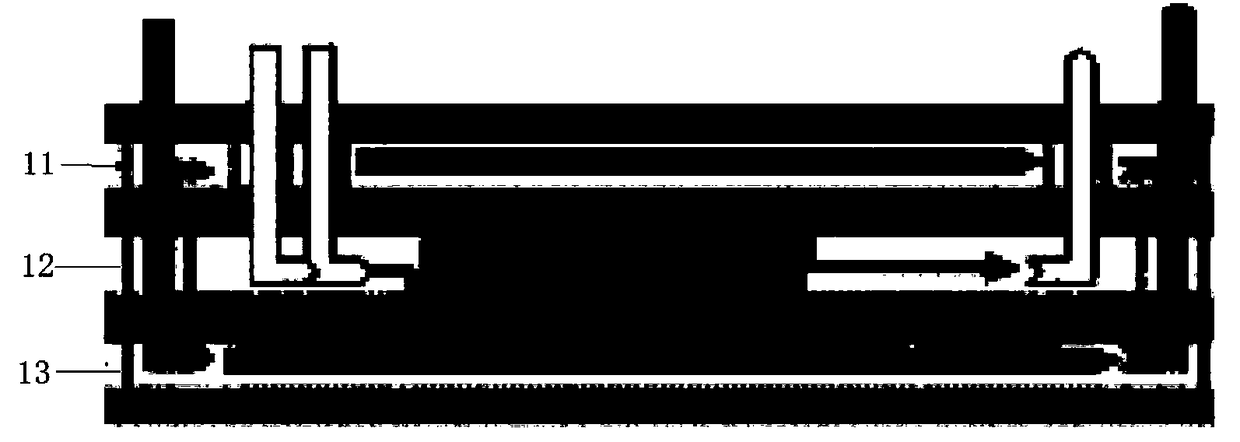
Continuous flow production process for etoricoxib intermediate
A production process, relying on the technology of Coxib, which is applied in chemical/physical/physicochemical processes, organic chemistry, chemical/physical processes, etc., can solve the problems of high material cost, high reaction heat, and high cost, and achieve novel catalysts and high reaction Mild conditions, relatively mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Synthesis of 1-(6-methylpyridin-3-yl)-2-(4-methylthiophenyl)ethanone:
[0046]In a continuous flow reactor, the first material input port is input (4-(methylthio)phenylacetic acid tetrahydrofuran solution (1mol / L concentration, 1mL / s flow rate), heated to 60 degrees by a heat exchanger, The second material input port inputs the tetrahydrofuran solution (2mol / L concentration, 1.5mL / s flow velocity) of tert-butylmagnesium chloride and mixes with the solution shown in the first inlet, and installs gas overflow device. After the two kinds of solutions are mixed, in Pass through the heat exchanger of 60 degrees in 60 minutes, then input the tetrahydrofuran solution (5mol / L concentration, 0.18mL / s flow velocity) of 6-methyl-pyridine-3-formic acid methyl ester from the third material input port, and Pass through a 60-degree heat exchanger (gas overflow device must be installed) within 60 minutes, and concentrate to collect tetrahydrofuran, and concentrate until the output s...
Embodiment 2
[0056] 1. Synthesis of 1-(6-methylpyridin-3-yl)-2-(4-methylthiophenyl)ethanone:
[0057] In a continuous flow reactor, the first material input port is input (4-(methylthio)phenylacetic acid in 2-methyltetrahydrofuran solution (1mol / L concentration, 1mL / s flow rate), heated by a heat exchanger To 70 degrees, the second material input port input 2-methyl tetrahydrofuran solution of tert-butylmagnesium chloride (2mol / L concentration, 2mL / s flow rate) and mix with the solution shown in the first inlet, and install a gas overflow device. After the two solutions were mixed, they passed through a heat exchanger of 70 degrees in 40 minutes, and then imported the 2-methyltetrahydrofuran solution (5mol / L concentration, 0.18mL / s flow rate), and pass through a heat exchanger at 70 degrees within 40 minutes (gas overflow device must be installed).
[0058] The fourth material input port is input with 10% dilute hydrochloric acid (0.35mL / s), and a gas overflow device must be installed. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com