Synthesis method of etoricoxib
A technology of etoricoxib and a synthesis method, applied in the field of synthesizing etoricoxib, can solve the problems of easy deterioration of sodium methoxide, large loss, flammability and explosion, etc., and achieves simplified post-processing process, improved synthesis steps, simplified reaction operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
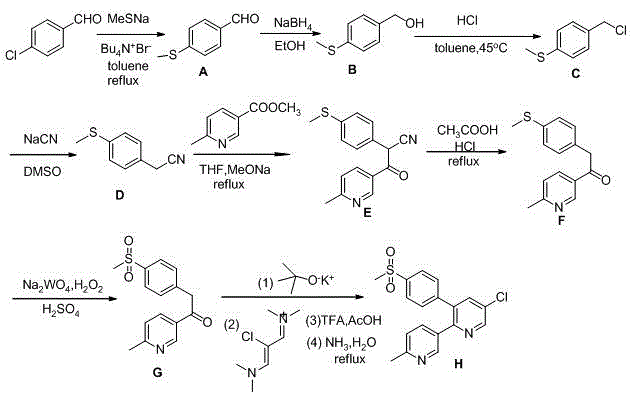
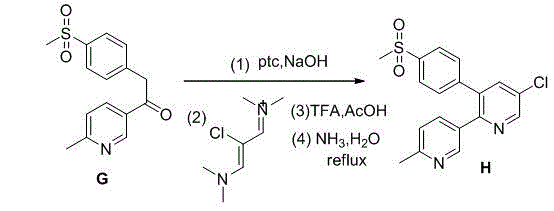
[0028] Chemical name of the following compound A: p-methylthiobenzaldehyde; chemical name of compound B: p-methylthiobenzyl alcohol; chemical name of compound C: p-methylthiobenzyl chloride; chemical name of compound D: p-methylthiobenzyl alcohol Benzyl cyanide; chemical name of compound E: 2-(4-methylthiophenyl)-3-(6-methylpyridinium)-3-yl-3-oxopropionitrile; chemical name of compound F: 3-p-methylthiophenylacetyl-6-picoline; Chemical name of compound G: 3-p-methylsulfonylphenylacetyl-6-picoline; Chemical name of compound G 2: (Z)-N -(2-Chloro-3-(dimethylimino)allylidene)-N-methylmethylamine; compound H is etoricoxib.
[0029] 1. Existing synthetic technology steps:
[0030] 1. Synthetic Compound A:
[0031] Raw materials: p-chlorobenzaldehyde: 14.6g; sodium methyl mercaptide: 50g (20% aqueous solution); tetrabutylammonium bromide: 3.5g; water: 7ml; toluene: 30ml.
[0032] Dissolve the starting material in toluene, add sodium methyl mercaptide, water, tetrabutylammonium br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com