Novel crystal form of salts prepared from etoricoxib and p-toluene sulfonic acid and preparation method thereof
A technology of p-toluenesulfonic acid and etoricoxib, which is applied in the fields of sulfonate preparation, organic chemistry methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
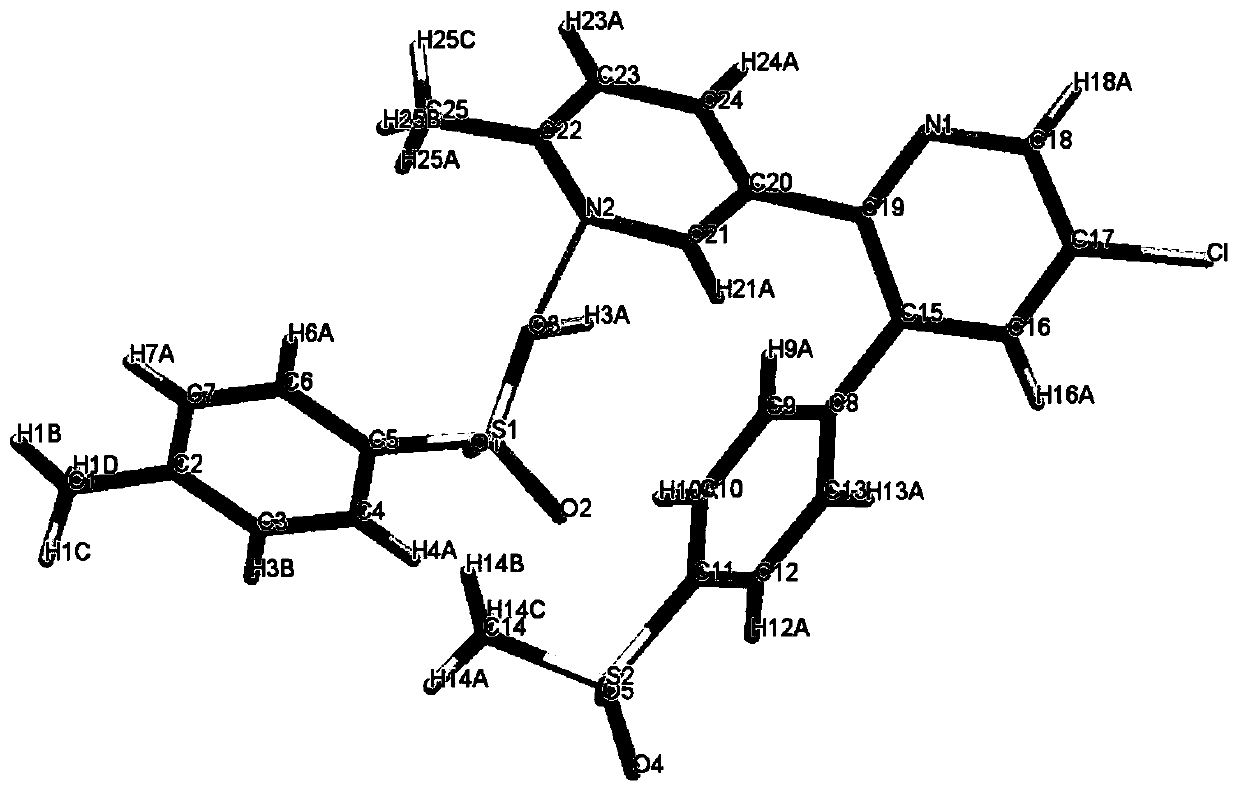
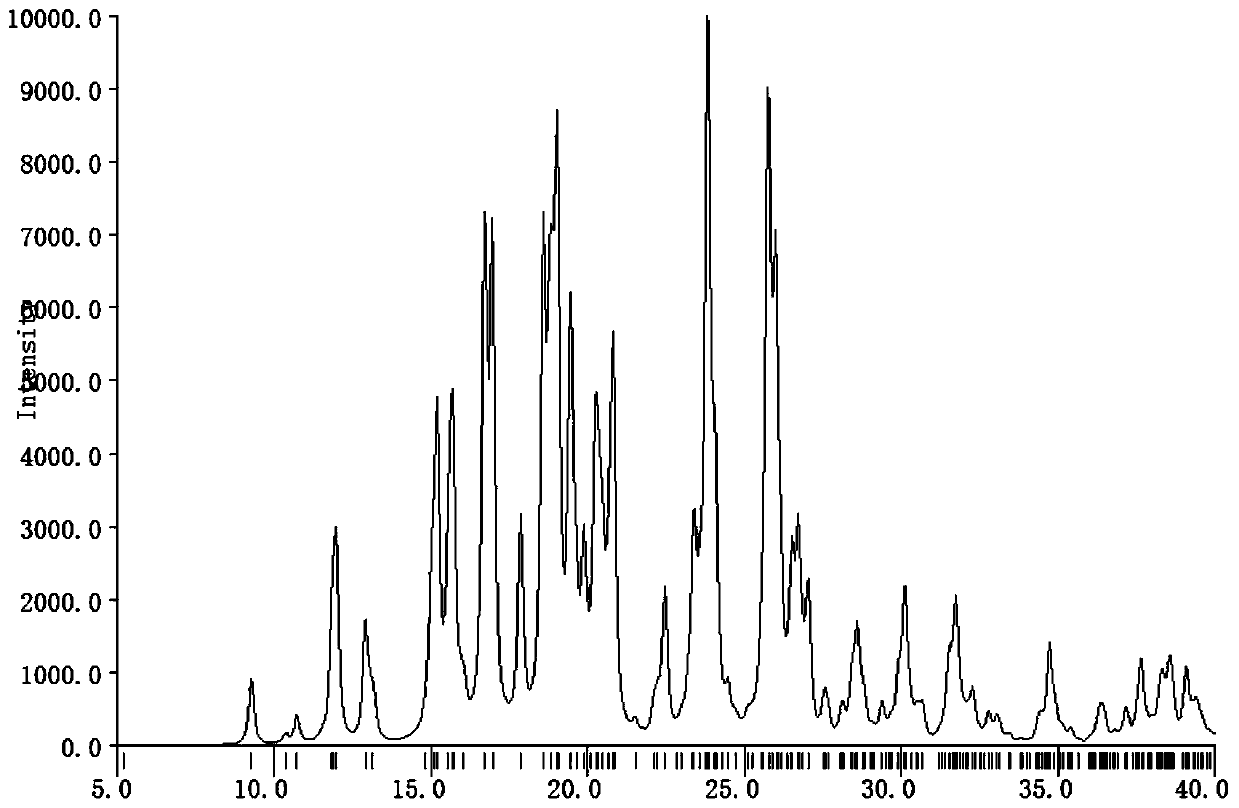
[0019] Example 1 Preparation of etoricoxib and p-toluenesulfonic acid to form a salt
[0020] Under ice-cooling, etoricoxib (358mg, 1mmol) and p-toluenesulfonic acid (0.8mmol) were added in a 50ml Erlenmeyer flask, and 20ml of methanol was added in batches, and after stirring for half an hour, stop stirring when the solution was slightly turbid. , placed in an explosion-proof refrigerator at 10°C, slowly precipitated, filtered to remove excess solvent, washed, and vacuum-dried to obtain the product.
Embodiment 2
[0021] Example 2 Preparation of etoricoxib and p-toluenesulfonic acid to form a salt
[0022] Under ice bath, add etoricoxib (350mg, 1mmol) and p-toluenesulfonic acid (1.2mmol) into a 50ml Erlenmeyer flask, add 20ml methanol in batches, stop adding when the solution is slightly turbid, and stir for half an hour Finally, place it in an explosion-proof refrigerator at 0°C for slow precipitation, filter to remove excess solvent, wash, and vacuum-dry to obtain the product.
Embodiment 3
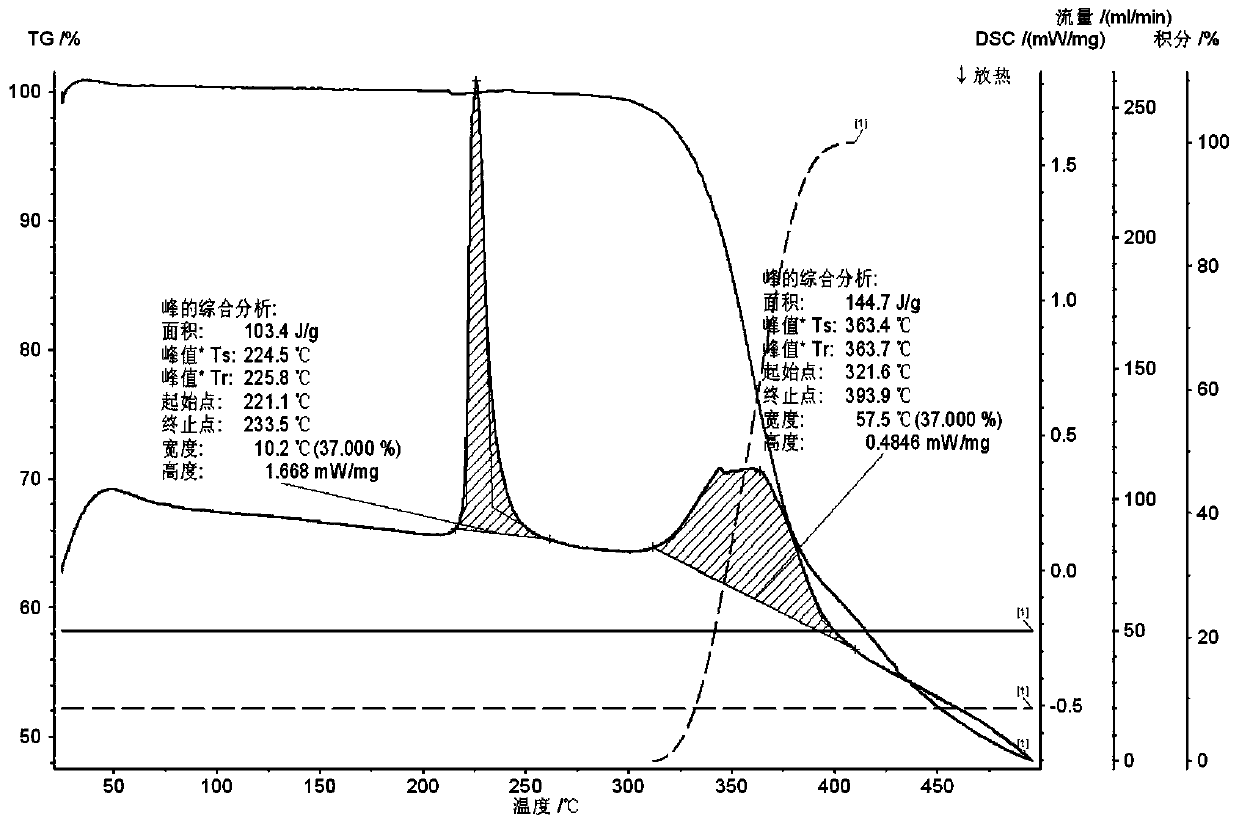
[0023] Embodiment 3 carries out high temperature test test to embodiment 1 gained salt crystal product
[0024] Two temperature levels of 40°C and 60°C were selected for the temperature respectively: the salt crystal sample obtained in Example 1 was placed at 60°C for 10 days, and samples were taken on the 5th and 10th days, and tested according to the key stability inspection items; if If there is no obvious change in the sample, the test at 40°C will not be carried out; if the sample has obvious changes (such as a 5% decrease in content, a large change in appearance and color if the identification is not obvious, etc.), the test must be carried out in the same way at 40°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com