Medicinal composition with etoricoxib and preparation method of medicinal composition
A technology of etoricoxib and composition, applied in the field of external skin preparations containing etoricoxib and preparation thereof, can solve problems such as decreased stability, poor solubility of etoricoxib, and inability to use effectively
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
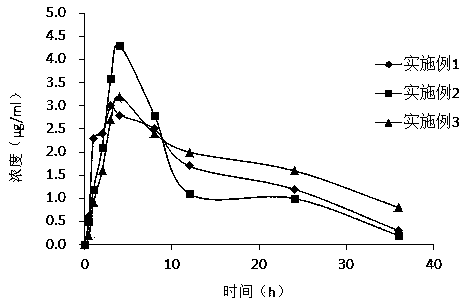
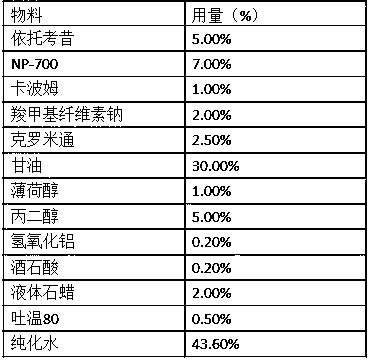
Embodiment 1
[0031]
[0032] Preparation:
[0033] 1) Add tartaric acid into pure water to obtain component A;
[0034] 2) Dissolve etoricoxib in the solution of propylene glycol and crotamiton, mix and stir evenly, component B;
[0035] 3) Weigh NP-700, carbomer, sodium carboxymethylcellulose, aluminum hydroxide, and Tween 80 into a kneading pot, add glycerin, and disperse evenly. Continue to add component B, menthol, and liquid paraffin, make it fully kneaded, and disperse evenly, then add component A, keep warm at about 40°C, mix, stir evenly, and make a paste;
[0036] 4) Apply the paste evenly, slice it, pack it, and make a patch.
Embodiment 2
[0038]
[0039] Preparation:
[0040] 1) Add gelatin to purified water, add tartaric acid after dissolving, and stir evenly to obtain component A;
[0041]2) Dissolve etoricoxib in a solution of crotamiton, nitrogen methylpyrrolidone and PEG400, mix and stir evenly to obtain component B;
[0042] 3) Weigh methylcellulose, carbomer, aluminum hydroxide, edetate disodium, and titanium dioxide into a kneading pot, add glycerin, and disperse evenly. Continue to add component B, azone, and liquid paraffin to fully knead and disperse evenly, then add component A, keep warm at about 40°C, mix, stir evenly, and make a paste;
[0043] 4) Apply the paste evenly, slice it, pack it, and make a patch.
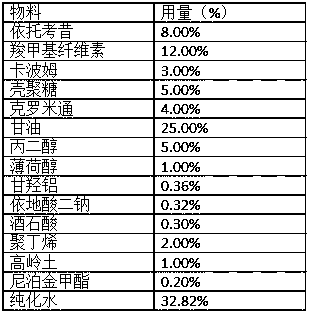
Embodiment 3
[0045]
[0046] Preparation:
[0047] 1) Add tartaric acid into purified water and stir evenly to obtain component A;
[0048] 2) Dissolve etoricoxib in a solution of propylene glycol and crotamiton, mix and stir evenly to obtain component B;
[0049] 3) Weigh carboxymethyl cellulose, carbomer, chitosan, aluminum glycylate, disodium edetate, kaolin and methylparaben into a kneading pot, add glycerin, and disperse evenly. Continue to add component B, menthol and polybutene to make it fully kneaded and dispersed evenly, then add component A, keep warm at about 40°C, mix, stir evenly, and make a paste;
[0050] 4) Apply the paste evenly, slice it, pack it, and make a patch.
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com