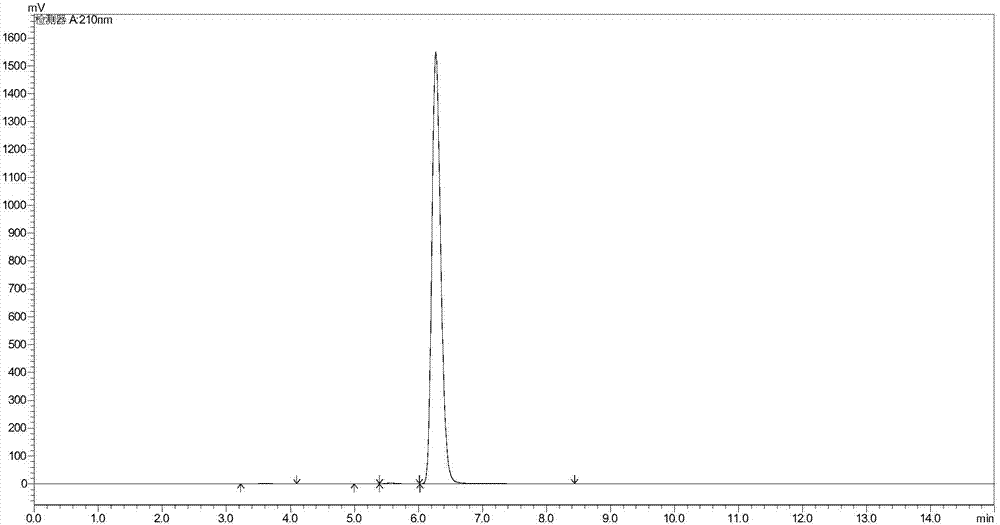
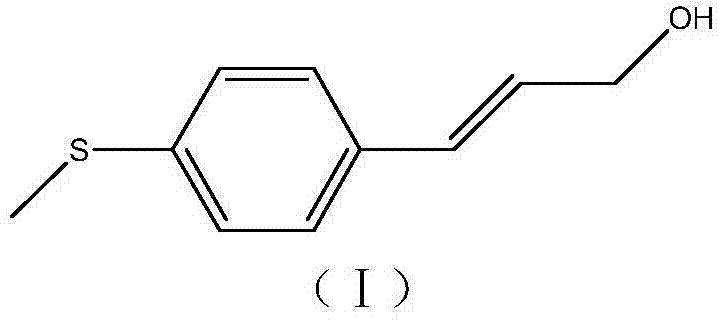
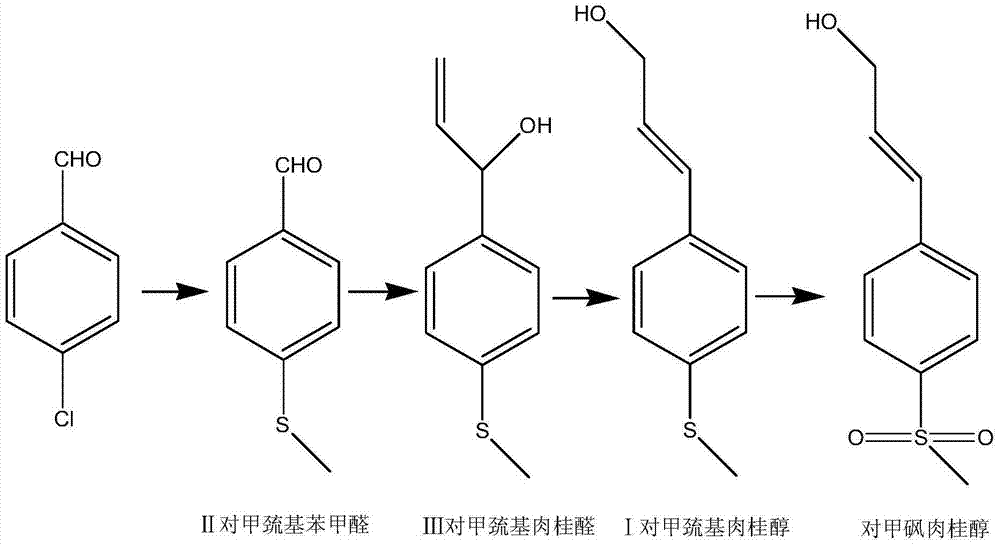
Simple synthetic method of trans-p-methylthiocinnamyl alcohol used for industrial production
A technology of methylmercaptocinnamyl alcohol and a synthetic method, which is applied in the field of medicinal chemistry, can solve problems such as difficult control of particularly smelly methylmercaptan, high potential safety hazards, and high price, and achieve easy operation of reaction conditions, cheap and easy-to-obtain raw materials, and reaction selection sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of p-methylmercaptobenzaldehyde
[0042] Add 124.0 g (1.0 mol) of sulfide anisole and 200 g of N,N-dimethylformamide into a 500 mL four-necked flask equipped with stirring, thermometer, reflux condensing device and dropping funnel, and cool to minus 10°C , Slowly add 161.1 g (1.05 moles) of phosphorus oxychloride dropwise, after dropping, react at 0°C for 2 hours, naturally rise to 20°C for 2 hours, then rise to 60-70°C for 2 hours. The reaction is over.
[0043] Cool down to 20°C, pour the reaction liquid into 1500g of ice-water mixture, adjust the pH value to 6-7 with 40% aqueous sodium hydroxide solution, extract three times with ethyl acetate (250g×3, 750g in total), and combine the organic phases , washed with 200 grams of saturated sodium chloride, layered, and 40 grams of anhydrous sodium sulfate dried the organic phase. After precipitation, 144.5 grams of light yellow liquid p-methylmercaptobenzaldehyde was obtained. The gas phase ...
Embodiment 2
[0044] Embodiment 2: Preparation of p-methylmercaptobenzaldehyde (II)
[0045]Add 124.0 g (1.0 mol) of sulfide anisole and 100 g of N,N-dimethylformamide into a 500 mL four-necked flask equipped with stirring, thermometer, reflux condensing device and dropping funnel, and cool to -10°C , slowly add dropwise a solution containing 109 grams (1.1 moles) of solid phosgene and 300 grams of 1,2-dichloroethane, after the drop is completed, react at 0°C for 2 hours, then naturally rise to room temperature (20°C) for 2 hours, then Rise to 60-70°C for 2 hours. After the reaction is completed, cool down to 20°C, pour the reaction liquid into 1500 g of ice-water mixture, adjust the pH value to 6-7 with 40% aqueous sodium hydroxide solution, extract three times with 1,2-dichloroethane (200g×3, 600 grams in total), combined organic phases, washed with 200 grams of saturated sodium chloride, separated layers, dried the organic phases with anhydrous sodium sulfate (40 grams), obtained 145.9 ...
Embodiment 3
[0047] Embodiment 3: the preparation of trans-mercaptocinnamaldehyde
[0048] Add 300 grams of water and 10.0 grams of potassium acetate to a 1000 mL four-neck flask equipped with a stirring, thermometer and dropping funnel, heat up to 30°C, add 76.0 grams (0.5 moles) of p-mercaptobenzaldehyde and 66 grams of 40% ethyl alcohol dropwise. Aldehyde aqueous solution and the mixed solution of 200 grams of methanol, add about 3 hours. Thereafter, the reaction was carried out at 30°C for 2 hours. Adjust the pH value to 6 with dilute hydrochloric acid, extract three times with ethyl acetate (450 g in total), combine the organic phases, wash with 200 g of saturated sodium chloride, separate layers, dry the organic phase with anhydrous sodium sulfate (30 g), and remove the solvent Obtain 82.8 grams of light yellow liquid, which is trans-mercaptocinnamaldehyde, with a gas phase purity of 98.9% and a yield of 93.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com