Method for synthesizing selenized benzothiophene compound
A benzothiophene and a synthesis method technology are applied in the field of selenized benzothiophene compounds and their synthesis, and can solve the problems of poor functional group tolerance, complicated operation of selenized benzothiophene compounds and the like, and achieve easy acquisition, wide reaction substrates, Simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] In the preparation method of the present invention, the reaction temperature is 80°C-130°C, preferably 100°C.
[0037] (4) Response time
[0038] In the preparation method of the present invention, the reaction time is not particularly limited. For example, the appropriate reaction time can be determined by detecting the target product or the residual percentage of the raw material through a gas chromatograph, which is usually 12-24 hours. A non-limiting example is 12 hours, 18 hours, 24 hours, the reaction time is preferably 12 hours.
[0039] In a preferred embodiment, the post-processing step after the reaction can be as follows: after the reaction, the reaction solution is cooled and then filtered with ethyl acetate, concentrated under reduced pressure, and the concentrate is separated by column chromatography (wherein the silica gel 300-400 mesh silica gel), using the mixture of petroleum ether and diethyl ether as the eluent, collecting the eluate, and concentrat...
Embodiment 1
[0043] Synthesis of 2-phenyl-3-(phenylselenyl)benzothiophene
[0044]
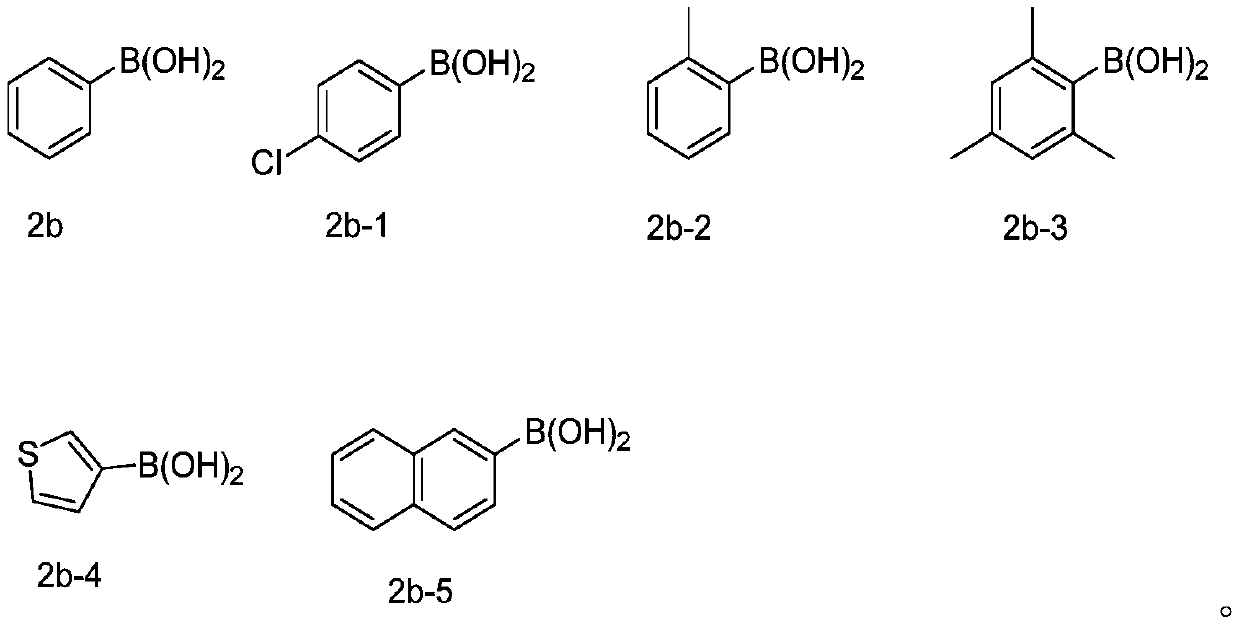
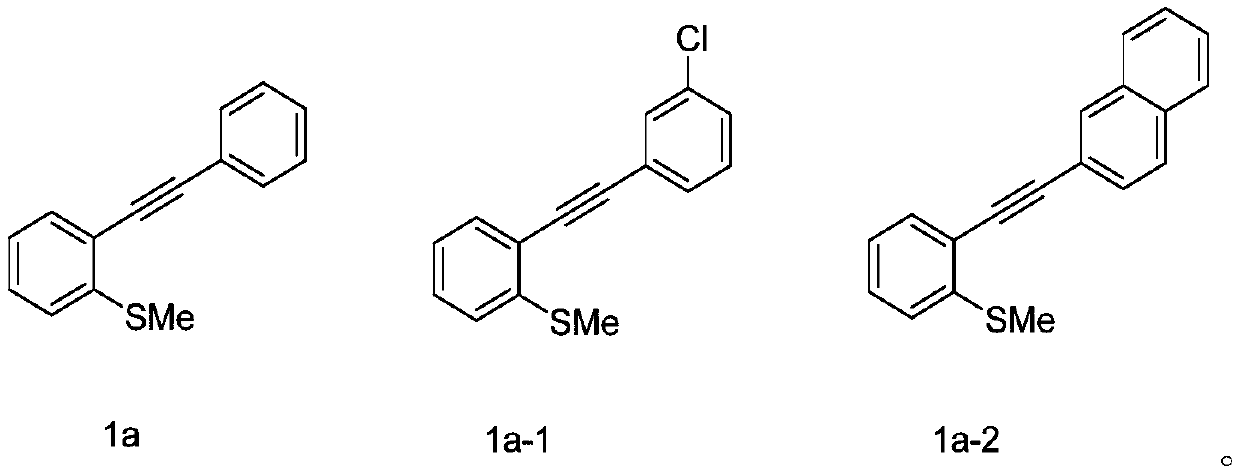
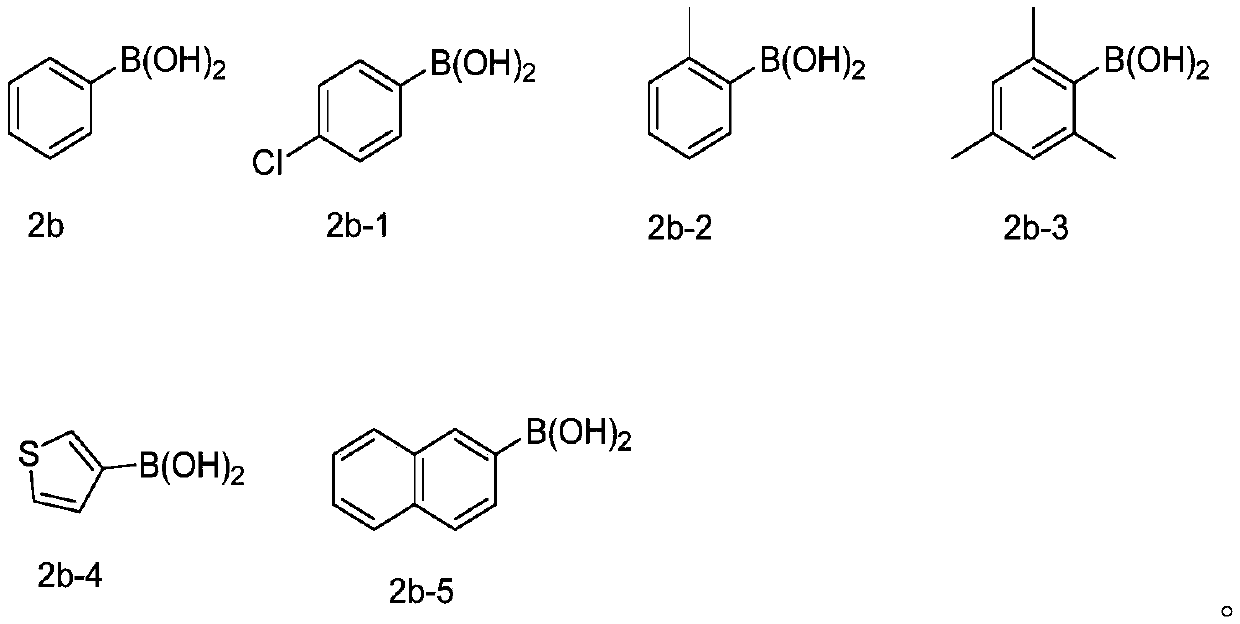
[0045] At room temperature, 2-phenylethynyl thioanisole (0.2mmol, 1equiv) of the structure shown in formula 1a, phenylboronic acid (0.4mmol, 2equiv) of the structure shown in formula 2b, selenium powder (0.4mmol, 2equiv) and Silver nitrite (0.04mmol, 0.2equiv) was added to the reaction tube, then pumped and replaced with oxygen three times, 2mL (282mmol) of DMSO was added, and stirred at a reaction temperature of 100°C for 12h. After the end of the reaction was monitored by thin layer chromatography, the reaction mixture was cooled, then diluted with ethyl acetate (the volume ratio of ethyl acetate to the reaction solution was 5:1), concentrated under reduced pressure to obtain a reaction concentrate, and the reaction concentrate was passed through the column Chromatographic separation, using petroleum ether as the eluent, collecting the eluent, spinning off the solvent to obtain the product, the produc...
Embodiment 2
[0052] Synthesis of 2-phenyl-3-(4-chlorophenylselenyl)benzothiophene
[0053]
[0054] At room temperature, 2-phenylethynyl anisole (0.2 mmol, 1 equiv) of the structure shown in formula 1a, 4-chlorophenylboronic acid (0.4 mmol, 2 equiv) of the structure shown in formula 2b-1, selenium powder (0.4 mmol, 2 equiv) and silver nitrite (0.04 mmol, 0.2 equiv) were added to the reaction tube, then pumped and replaced with oxygen three times, 2 mL of DMSO was added, and stirred at a reaction temperature of 100 °C for 12 h. After the end of the reaction was monitored by thin layer chromatography, the reaction mixture was cooled, then diluted with ethyl acetate (the volume ratio of ethyl acetate to the reaction solution was 5:1), concentrated under reduced pressure to obtain a reaction concentrate, and the reaction concentrate was passed through the column Chromatographic separation, using petroleum ether as the eluent, collecting the eluent, spinning off the solvent to obtain the pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com