Method for synthesizing chiral sulfoxide from thioether under catalytic action of Rhodococcus
A technology for coccus-catalyzed thioether and Rhodococcus, applied in the field of microorganisms, can solve the problems that it is difficult to meet the high requirements for the purity of chiral natural products, the benzyl sulfoxide does not reach high optical purity, and achieves no by-products and conversion rate. High, mild reaction conditions, simple process route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
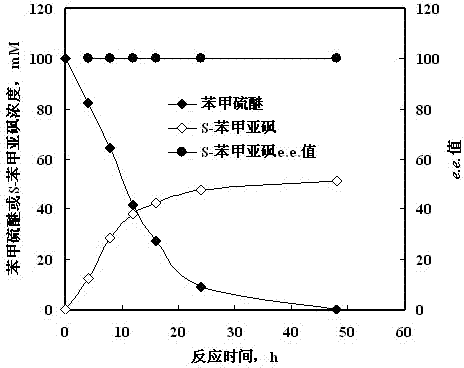
[0022] Rhodococcus sp. CCZU10-1 quiescent cells catalyze the synthesis of thioanisole S -Benzyl sulfoxide
[0023] Prepare medium (glucose 5 g, peptone 5 g, yeast extract 5 g, KH 2 PO 4 1 g, NaCl 0.1 g, MgSO 4 0.1 g, inducer sulfide anisole 0.01 g, water 1000 mL, pH 6.0), put 3 L medium in a 5 L shake flask, culture at 160 rpm and 25 °C for 48 h, centrifuge, Resting cells are obtained by washing. Weigh 0.1 g resting cells with a wet weight, suspend the cells in 1.0 mL pH 6.0 potassium phosphate buffer solution, add a final concentration of 100 mM sulfide anisole, and shake the reaction at 25 °C and 160 rpm for 48 h ( figure 1 shown), S - Benzyl sulfoxide yield 51.3%, e.e. >99.9%.
[0024]
Embodiment 2
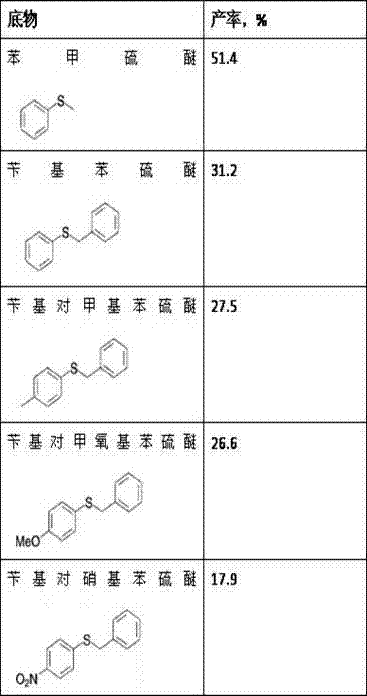
[0026] Rhodococcus Catalytic oxidation of different thioethers by sp. CCZU10-1 resting cells
[0027] Prepare medium (glucose 20 g, peptone 20 g, yeast extract 20 g, KH 2 PO 4 5 g, NaCl 1.5 g, MgSO 4 0.5 g, inducer sulfide anisole 10 g, water 1000 mL, pH 9.0), put 250 mL of culture medium in a 1 L shake flask, culture at 160 rpm and temperature 35 °C for 48 h, centrifuge, Resting cells are obtained by washing. Weigh 0.01 g of resting cells with a wet weight, suspend the cells in 1.0 mL of pH 8.0 potassium phosphate buffer solution, add different substrate thioethers to a final concentration of 20 mM, and place on a constant temperature shaker at 35 °C and 160 rpm Shaking reaction, after 12 h of reaction, the measurement results are shown in Table 1.
[0028] Table 1 Expansion of the substrate spectrum
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com