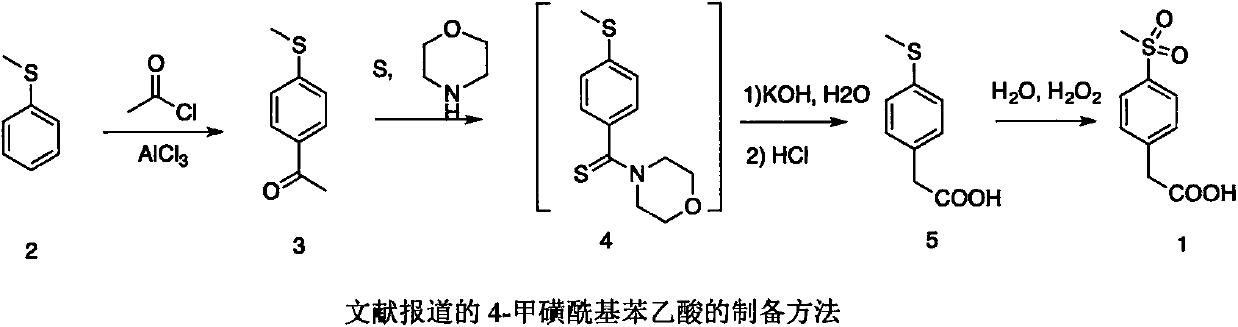
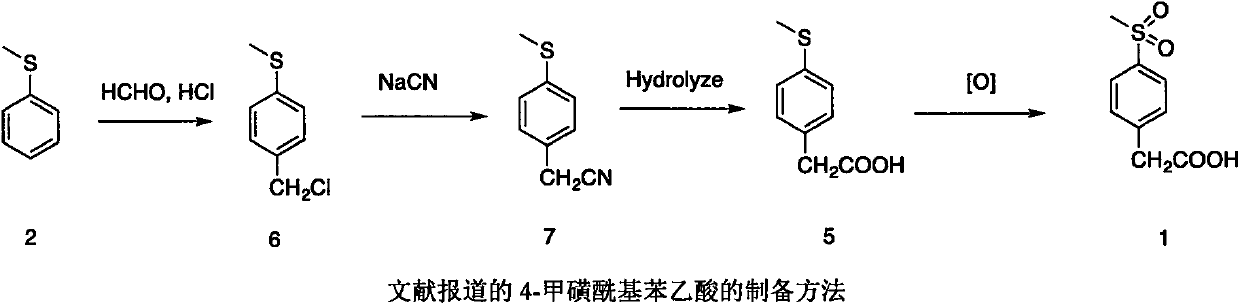
Preparation method of 4-methylsulphonylphenylacetic acid
A technology of methylsulfonylbenzene and acetic acid, which is applied in the field of synthesis of 4-methylsulfonylphenylacetic acid, can solve the problems of large environmental pollution, and achieve the effects of extensive material sources, personnel injury and environmental damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 4-Methylthiobenzaldehyde
[0030] Add 150mL of DMF and 100.0g of thioanisole to a 500mL dry three-necked flask, cool to -5°C -5°C, slowly add 150.0g of phosphorus oxychloride dropwise, and keep the internal temperature at -5°C to 5°C during the dropwise addition between. After the dropwise addition, slowly increase the reaction temperature to 35-45°C, stir the reaction for 7 hours, and detect the reaction by TLC. After the reaction was completely completed, the reaction liquid was cooled to room temperature, and the reaction liquid was slowly added to 500 mL of ice water, and the temperature was kept not higher than 35°C. After the transfer is completed, stir at no higher than 35°C for 10 minutes, add 200 mL of dichloromethane, adjust the pH to 7-8 with 10% sodium hydroxide, separate the organic phase, continue to extract once with 200 mL of dichloromethane, and combine The organic phase was dried with anhydrous sodium sulfate, filtered, the organic sol...
example 2
[0031] Example 2: Preparation of 4-methylthiophenylacetaldehyde
[0032] Add 50mL of methyl α-chloroacetate to a 500mL dry three-necked flask, add 100.0g of 4-methylthiobenzaldehyde, cool the reaction solution to 0-10°C, slowly add 40mL of 27-30% sodium methoxide solution, drop After the addition is completed, gradually increase the reaction temperature to 25-30°C, and stir the reaction at this temperature for 3 hours, add 50mL of 20% sodium hydroxide solution, increase the reaction temperature to 55-60°C, hydrolyze for 2 hours, and Next, hydrochloric acid was added dropwise, and a large amount of gas was generated during the dropwise addition. After adding dropwise until no gas was generated, the reaction solution was cooled to room temperature, extracted three times with 300 mL of ethyl acetate, and the organic layers were combined.
[0033] Add anhydrous magnesium sulfate to the organic layer to dry, filter, and distill. When 200 mL remains, add 500 mL of n-heptane, contin...
example 3
[0034] Example 3: Preparation of 4-methylthiophenylacetaldehyde
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com