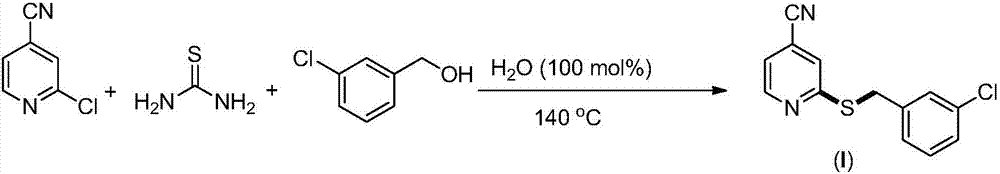
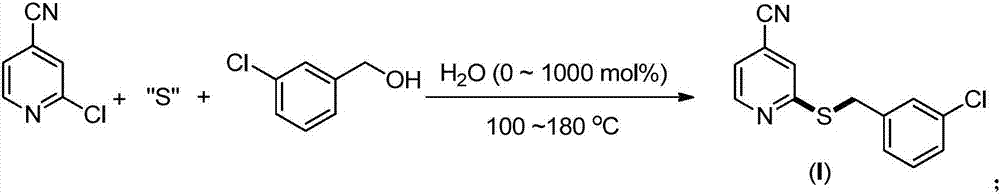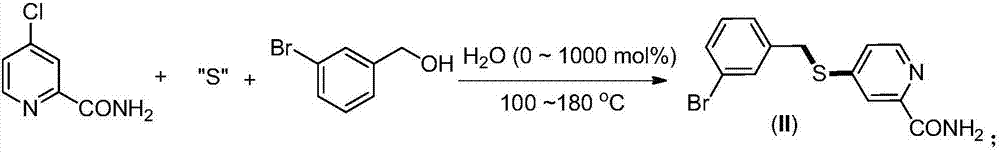Synthesis method of 2-(4-cyano) pyridyl (3-cyano) thioanisole
A technology of anisole sulfide and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of transition metal residue, low total yield, long reaction period, etc., and achieves easy operation, high reaction efficiency, and low requirements for reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of 2-(4-cyano)pyridyl(3-chloro)sulfide anisole (I)(0.50mmol) from 2-chloro-4-cyanopyridine, thiourea and 3-chlorobenzyl alcohol
[0024]
[0025] 2-Chloro-4-cyanopyridine (82.8mg, 0.60mmol, 1.2equiv.), thiourea (45.6mg, 0.60mmol, 1.2equiv.), 3-chlorobenzyl alcohol (71.0mg, 0.50mmol,) and water (9.0mg, 0.50mmol) were directly sealed under air, and then heated to 140°C for 24h under solvent-free conditions. After the completion of the reaction monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 70% (91.0 mg). 1 H NMR (400MHz, CDCl 3 )δ8.59(d,J=5.0Hz,1H),7.39(s,1H),7.37(s,1H),7.28–7.13(m,4H),4.41(s,2H); 13 C NMR (100MHz, CDCl 3 ) δ 160.4, 150.1, 139.4, 134.3, 129.8, 129.1, 127.6, 127.1, 123.7, 120.4, 116.2, 33.6.
Embodiment 2
[0027] Preparation of 2-(4-cyano)pyridyl(3-chloro)sulfide anisole (I)(10.0mmol) from 2-chloro-4-cyanopyridine, thiourea and 3-chlorobenzyl alcohol
[0028]
[0029] In the tubular reactor, 2-chloro-4-cyanopyridine (1.656g, 12.0mmol, 1.2equiv.), thiourea (0.912g, 12mmol, 1.2equiv.) and 3-chlorobenzyl alcohol (1.420g, 10mmol ,), sealed directly in the air, and then heated to 140°C for 24h under solvent-free conditions. After the completion of the reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 65% (1.692g). 1 H NMR (400MHz, CDCl 3 )δ8.59(d,J=5.0Hz,1H),7.39(s,1H),7.37(s,1H),7.28–7.13(m,4H),4.41(s,2H); 13 C NMR (100MHz, CDCl 3 ) δ 160.4, 150.1, 139.4, 134.3, 129.8, 129.1, 127.6, 127.1, 123.7, 120.4, 116.2, 33.6.
Embodiment 3
[0031] Preparation of 4-(2-formamido)pyridyl(3-bromo)sulfide anisole(II)(10.0mmol) from 4-chloropyridine-2-carboxamide, thiourea and 3-bromobenzyl alcohol
[0032]
[0033] 4-chloropyridine-2-carboxamide (1.872g, 12.0mmol, 1.2equiv.), thiourea (0.912g, 12mmol, 1.2equiv.) and 3-bromobenzyl alcohol (1.860g, 10mmol,), sealed directly in the air, and then heated to 140°C for 24h under solvent-free conditions. After the completion of the reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 33% (1.054g). 1 H NMR (400MHz, CDCl 3 )δ8.33(d,J=5.0Hz,1H),8.08(d,J=1.5Hz,1H),7.86(brs,1H),7.57(s,1H),7.42(d,J=8.0Hz, 1H), 7.34(d, J=8.0Hz, 1H), 7.27–7.16(m, 3H), 5.76(s, 1H), 4.24(s, 2H). 13 C NMR (100MHz, CDCl 3 ) δ 166.2, 150.8, 149.1, 147.7, 137.4, 131.8, 131.0, 130.4, 127.5, 123.1, 122.8, 119.1, 35.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com