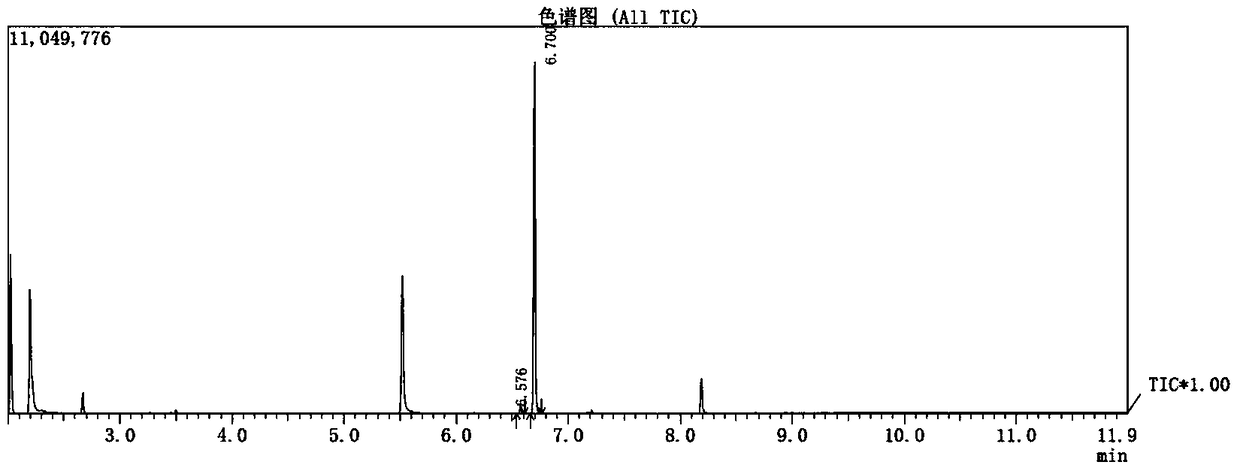
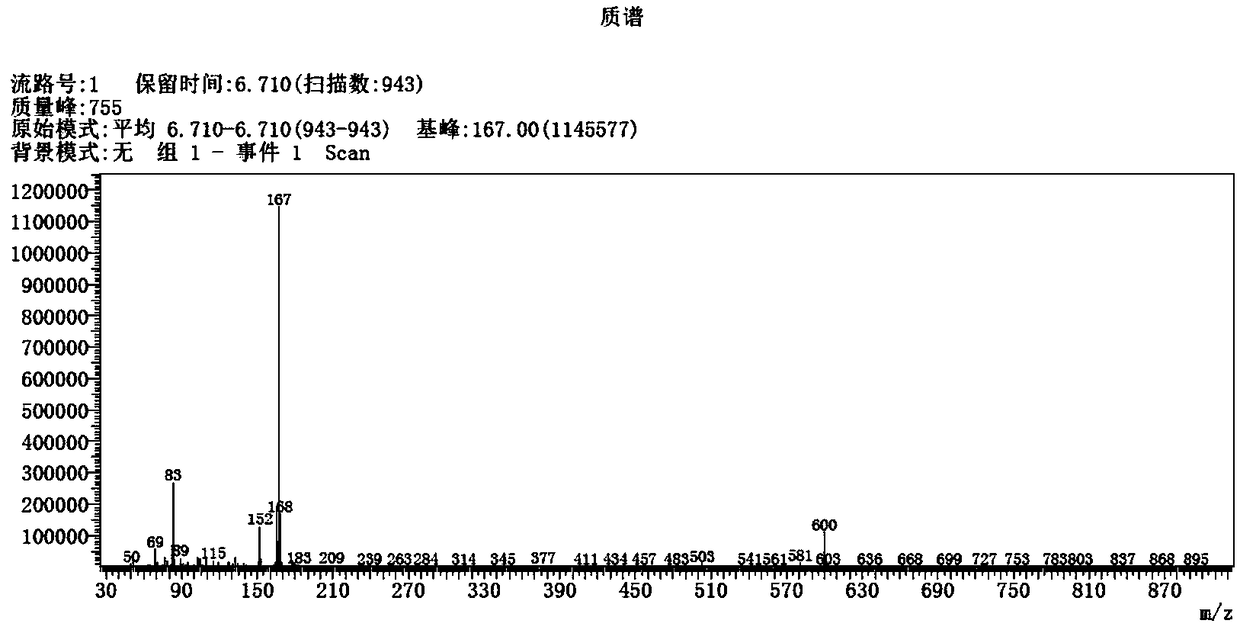
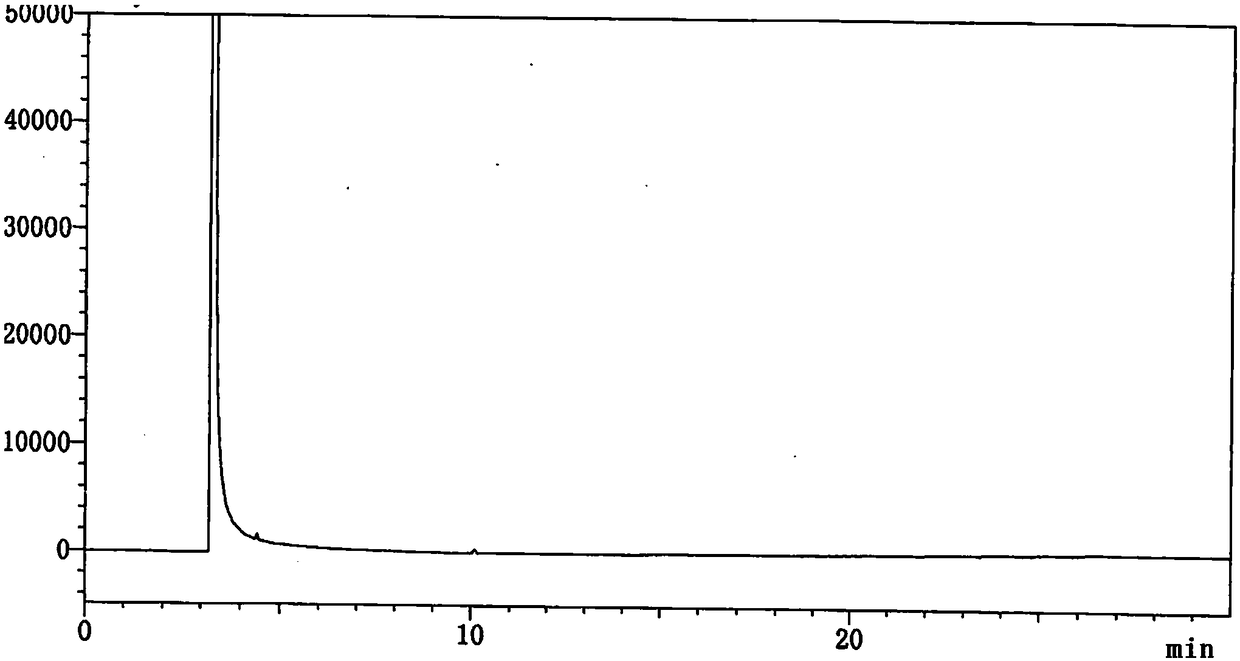
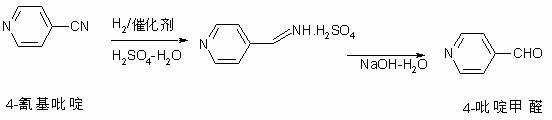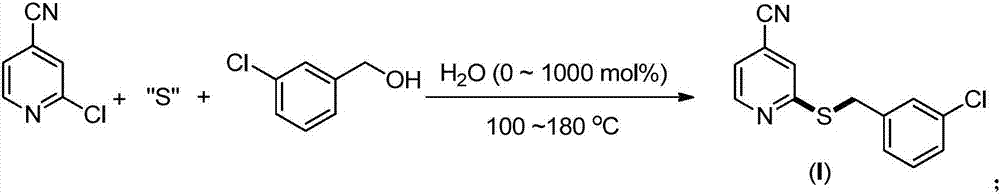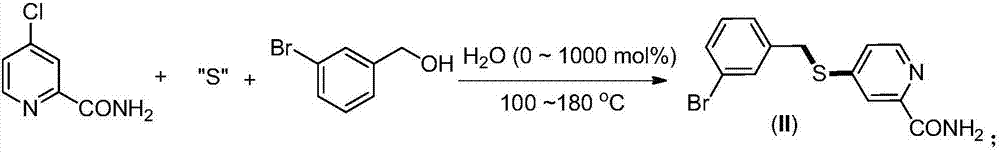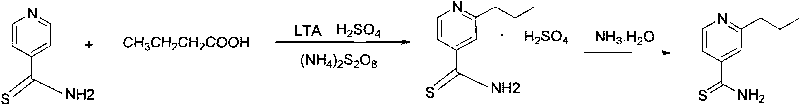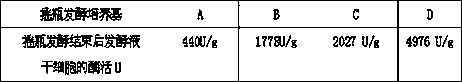Patents
Literature
41 results about "4-cyanopyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
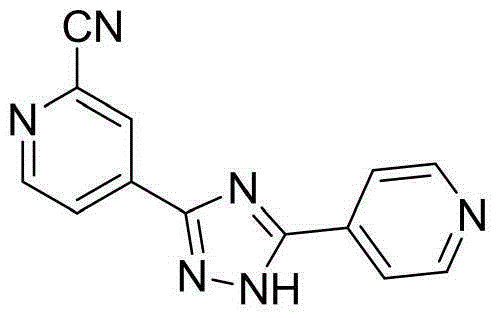
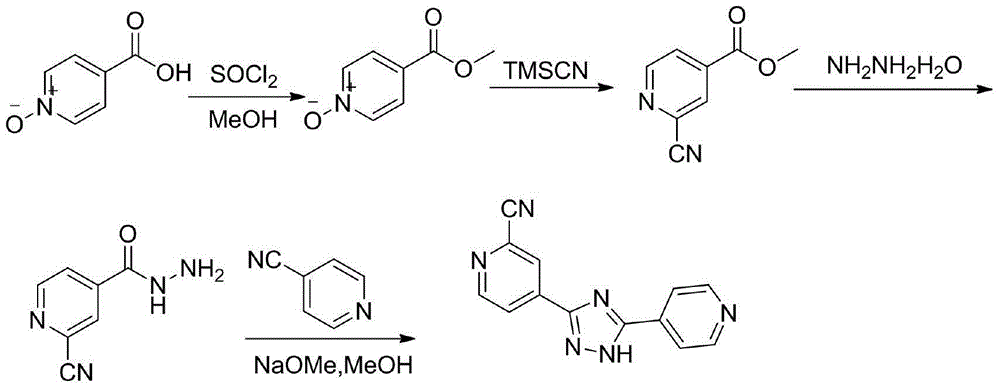
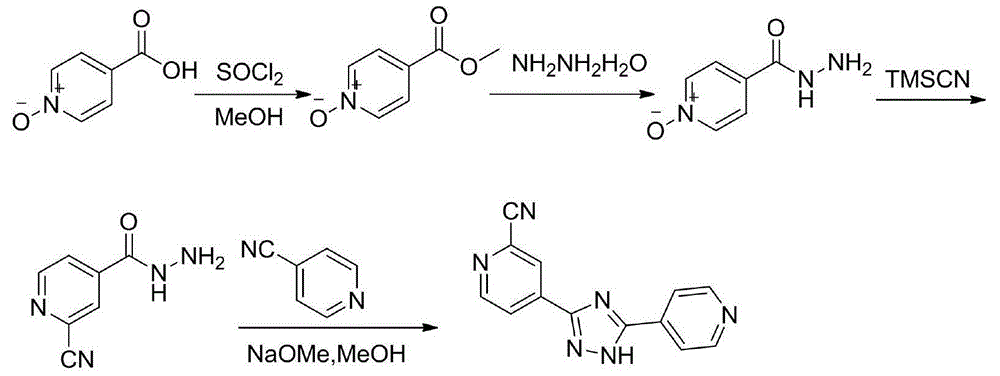
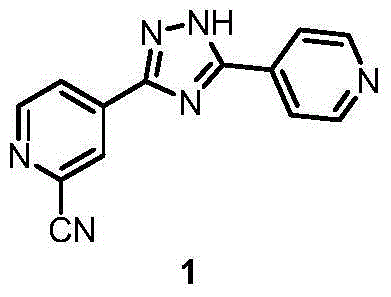
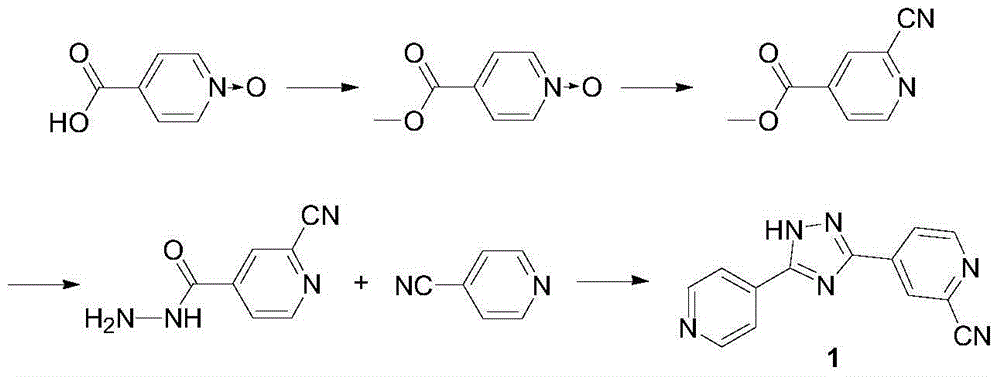
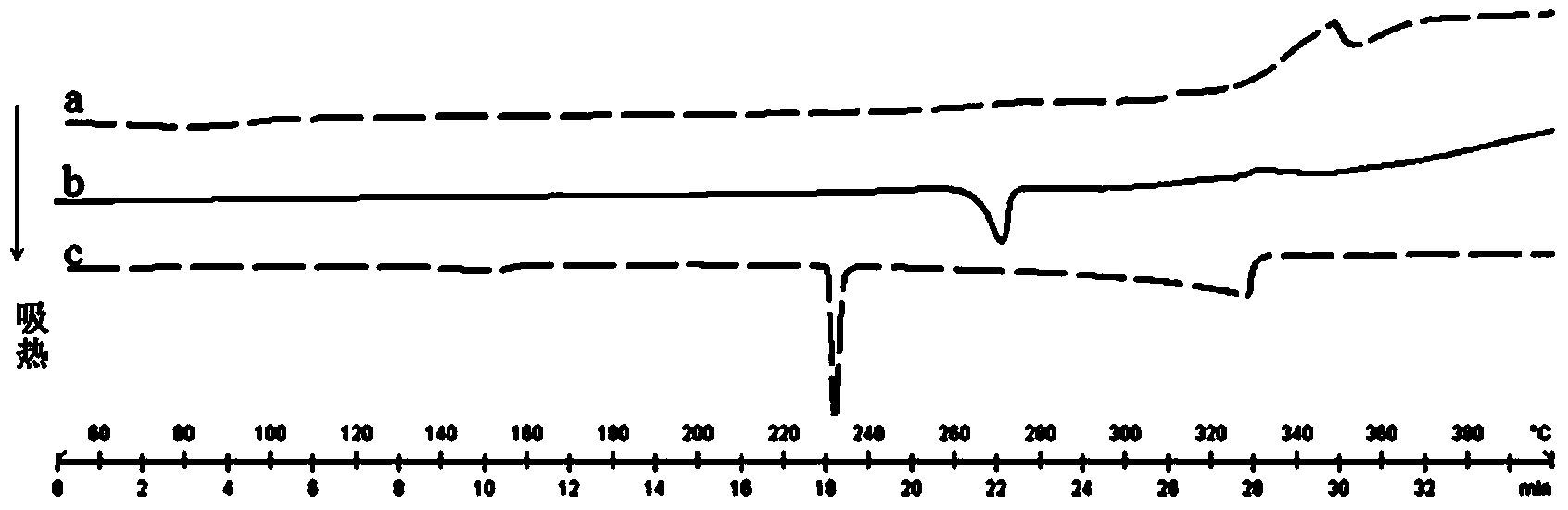
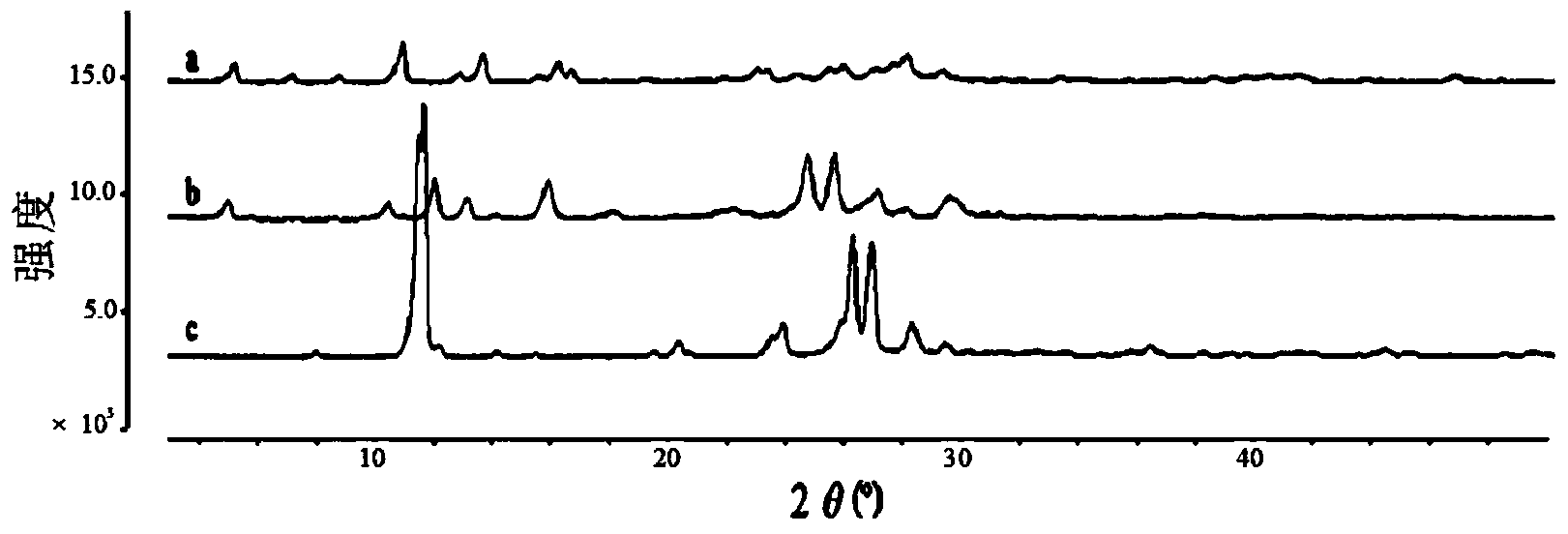
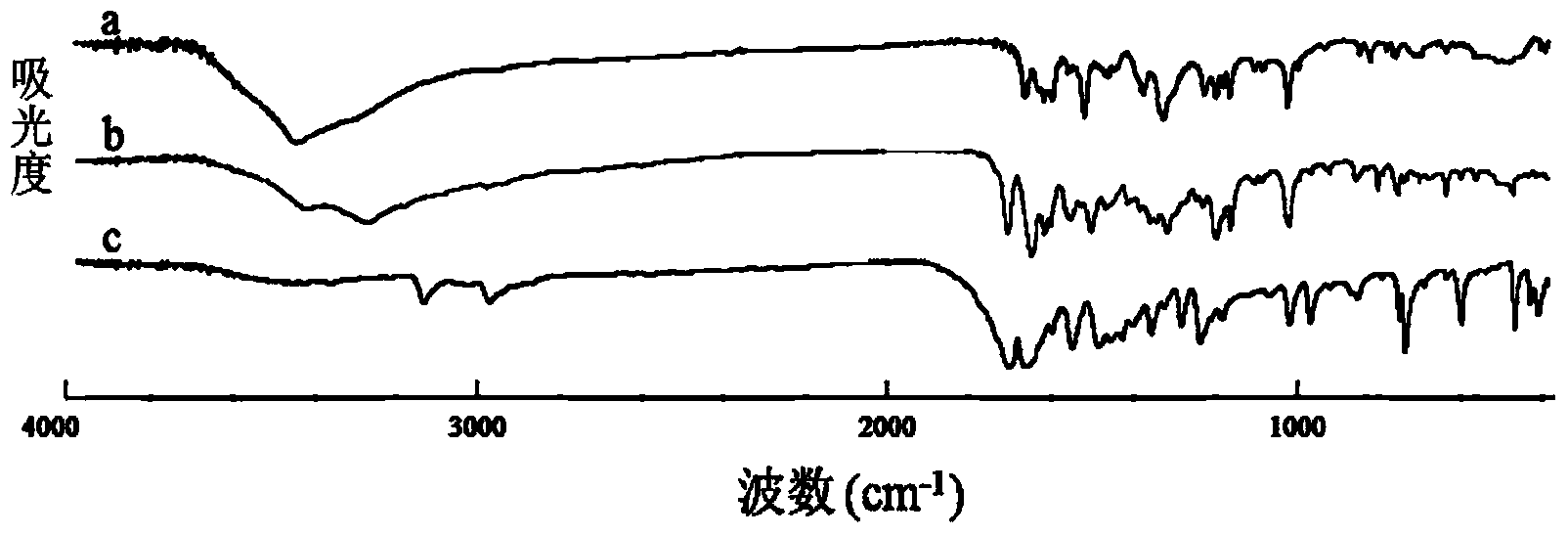
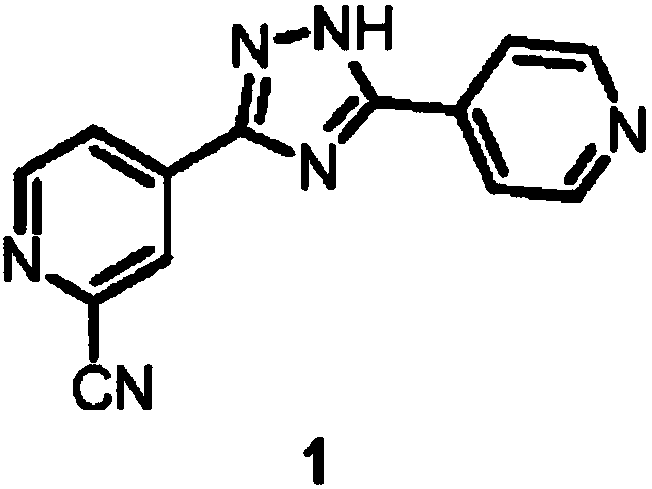
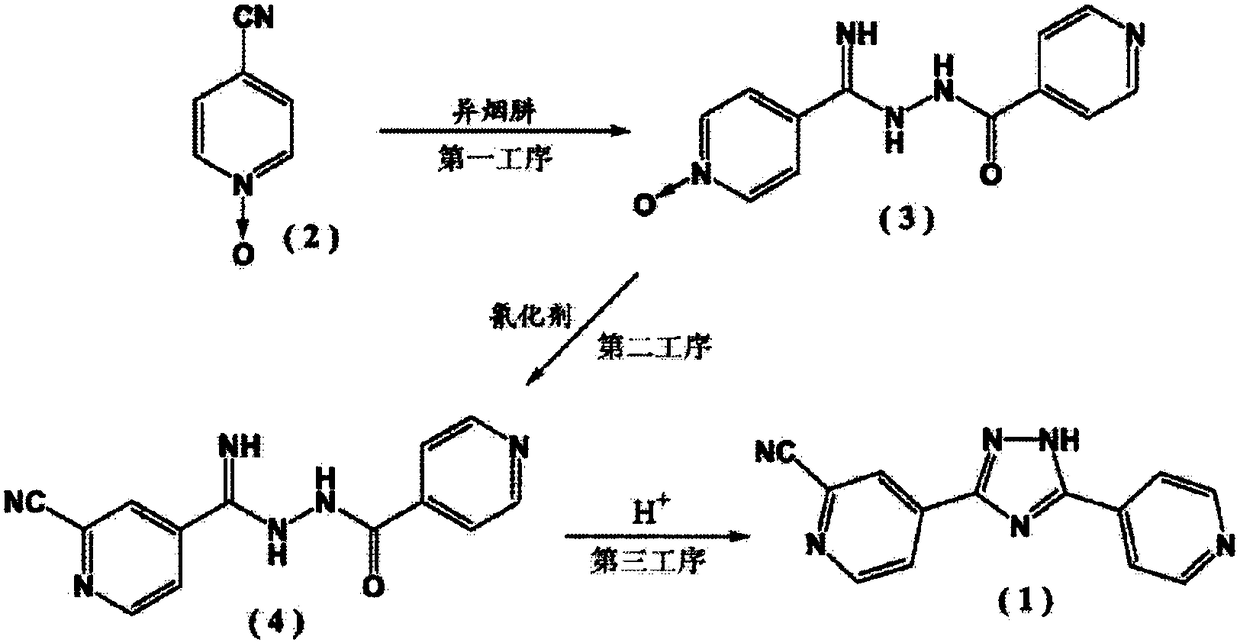
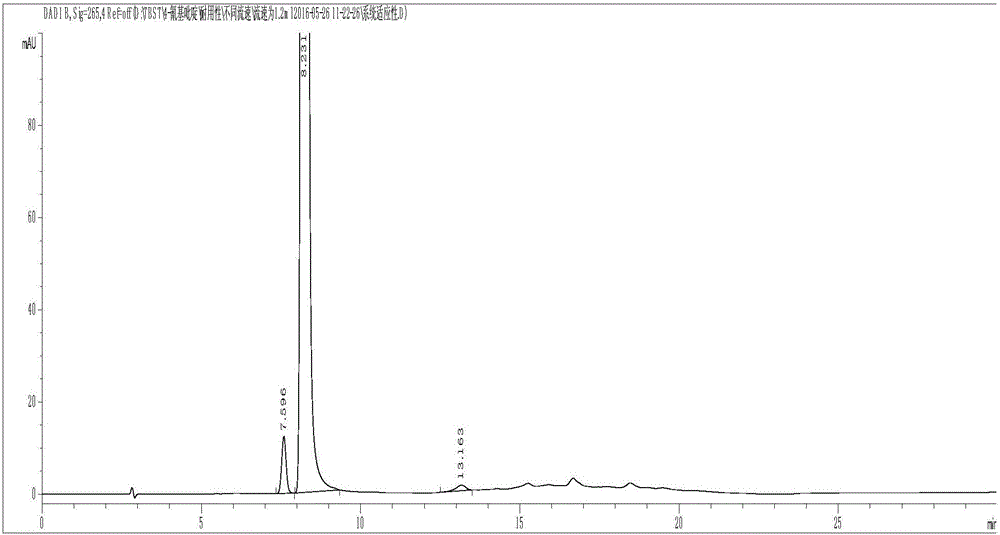
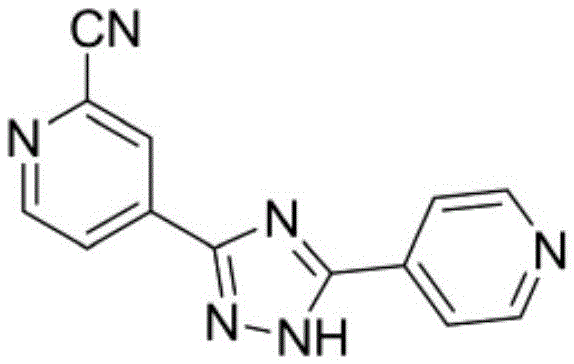
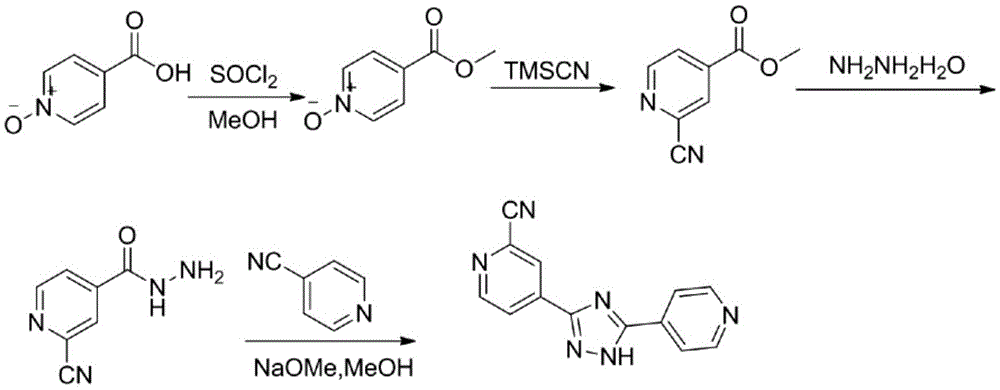
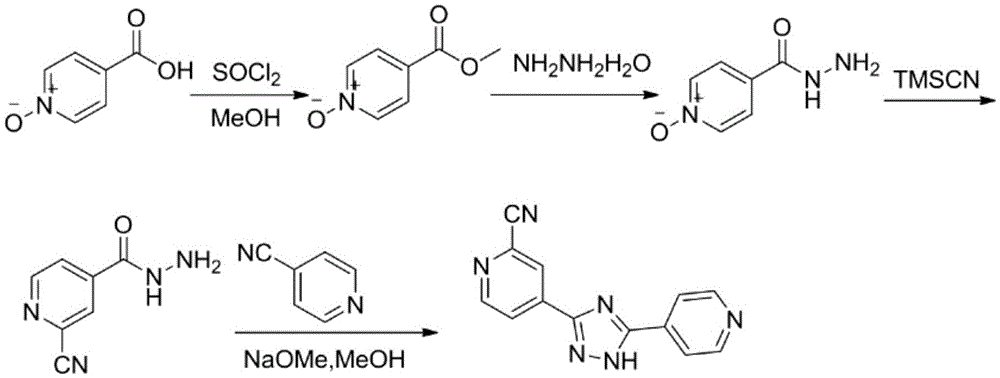
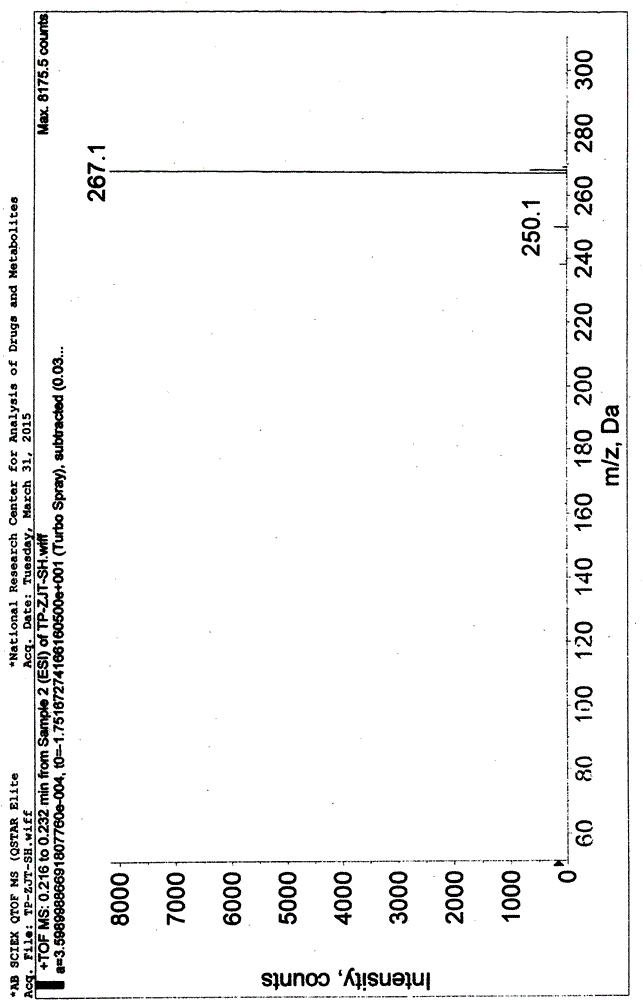
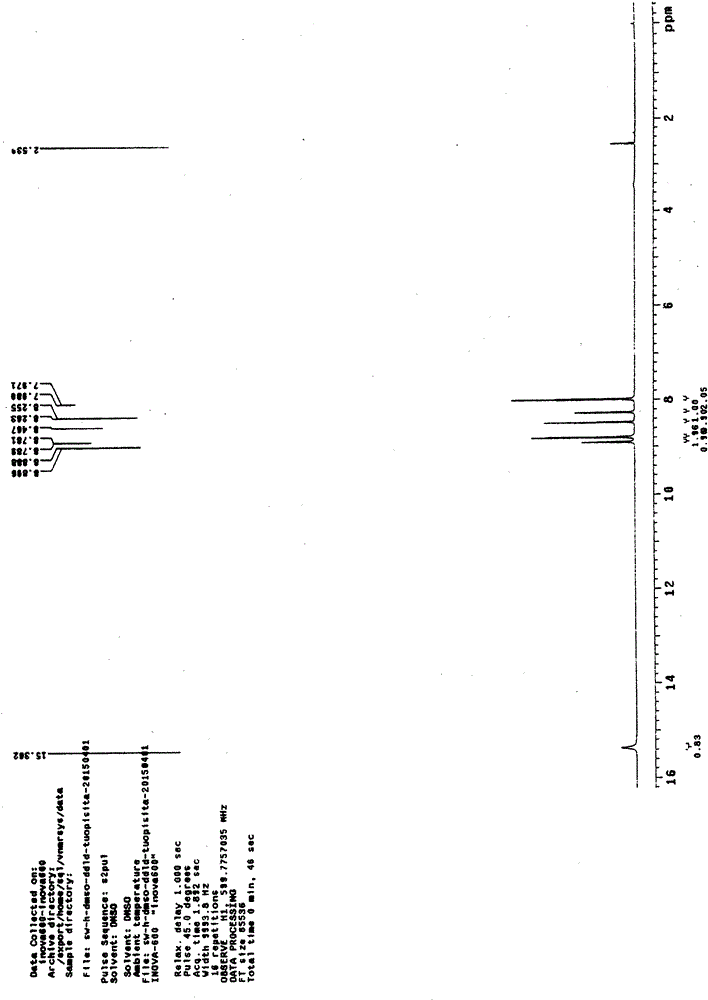
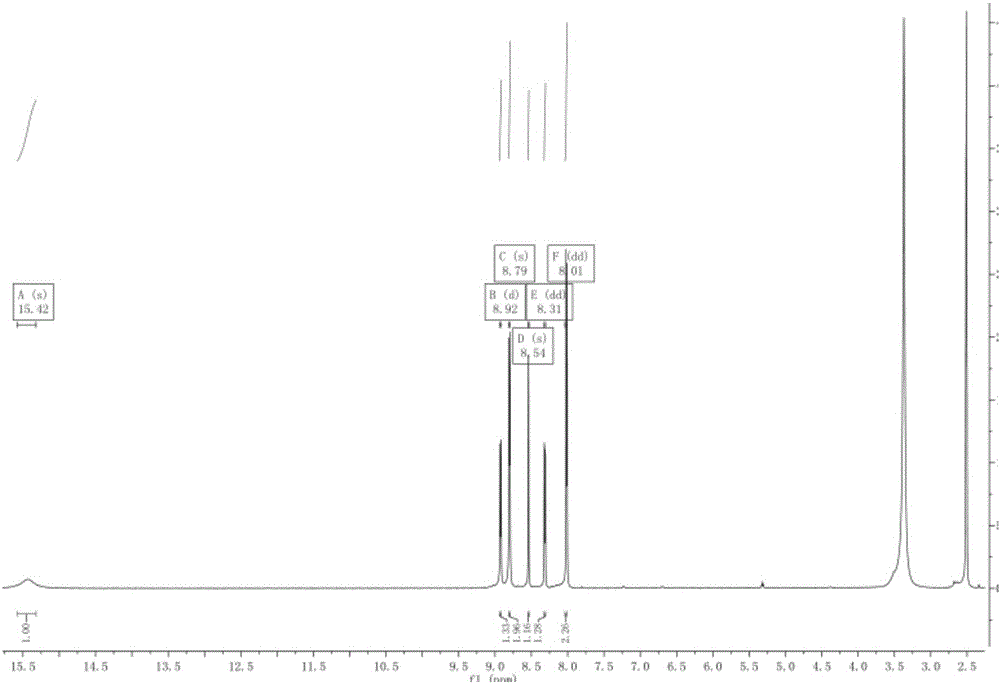
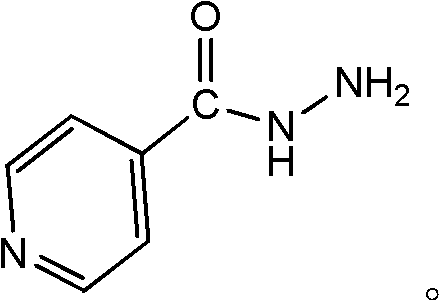
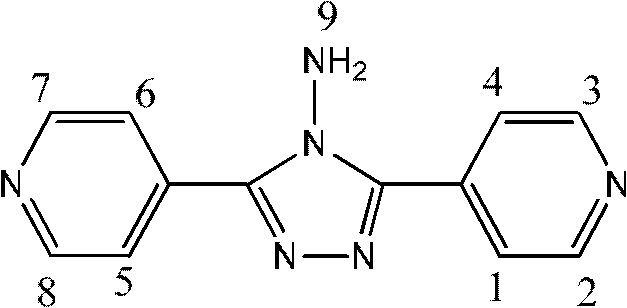
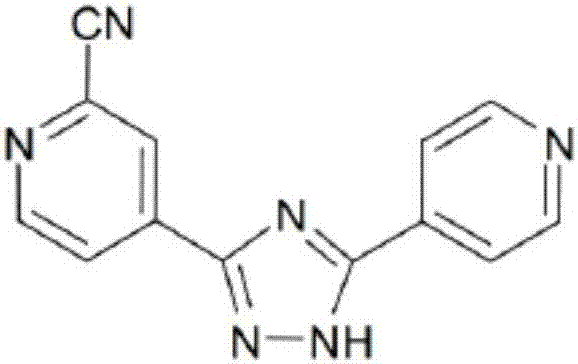
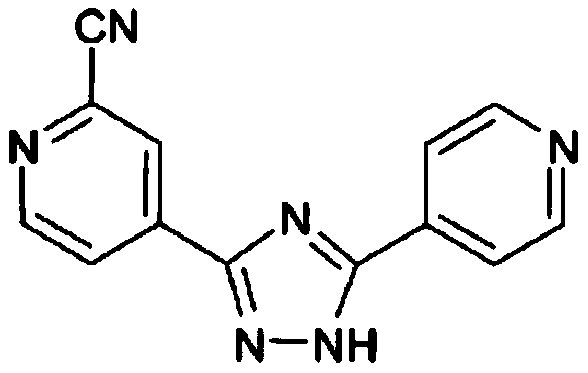
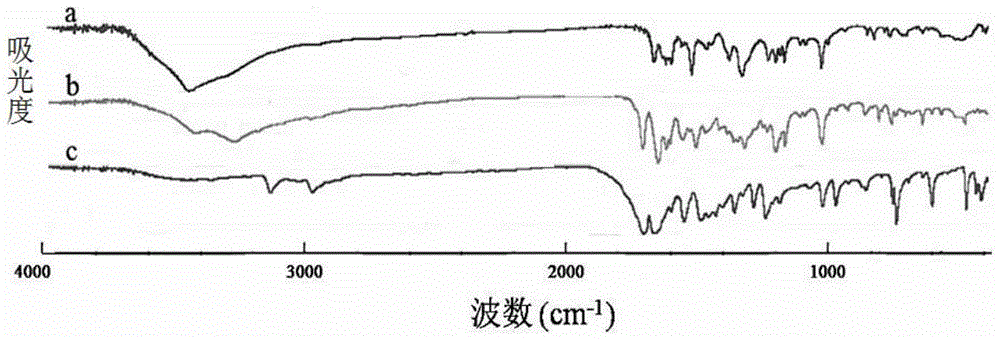
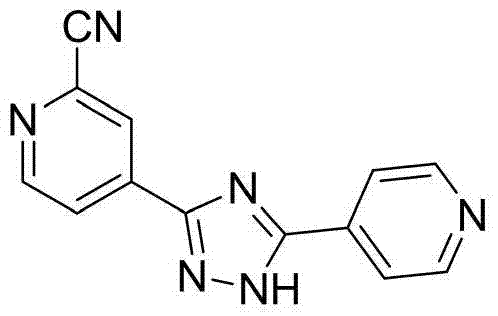
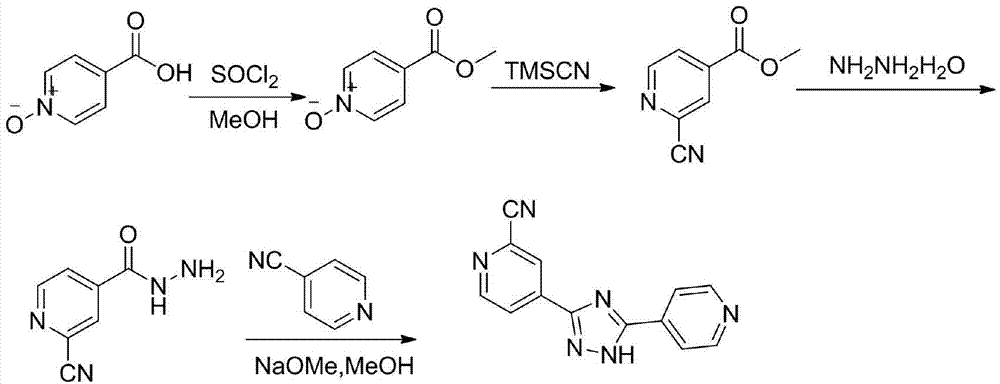
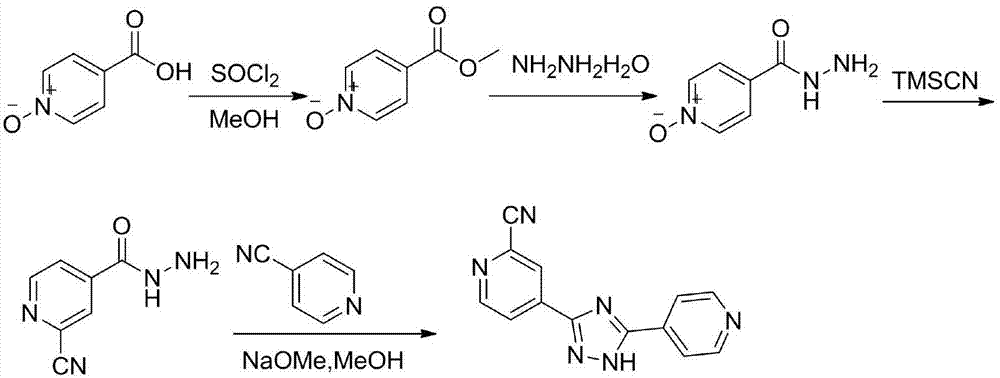
Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile
InactiveCN104151297AEasy to operateSimple processing capacityOrganic chemistryTopiroxostatDimethylcarbamyl chloride
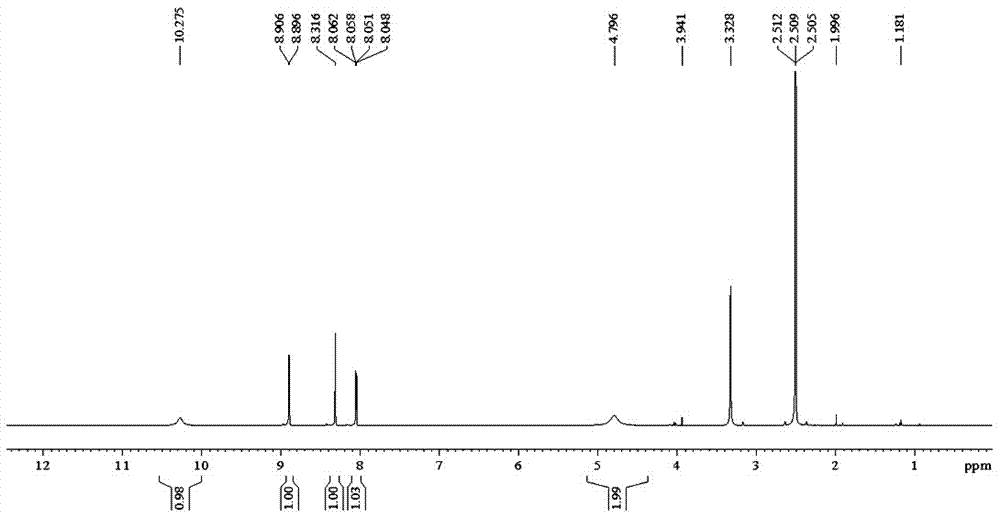
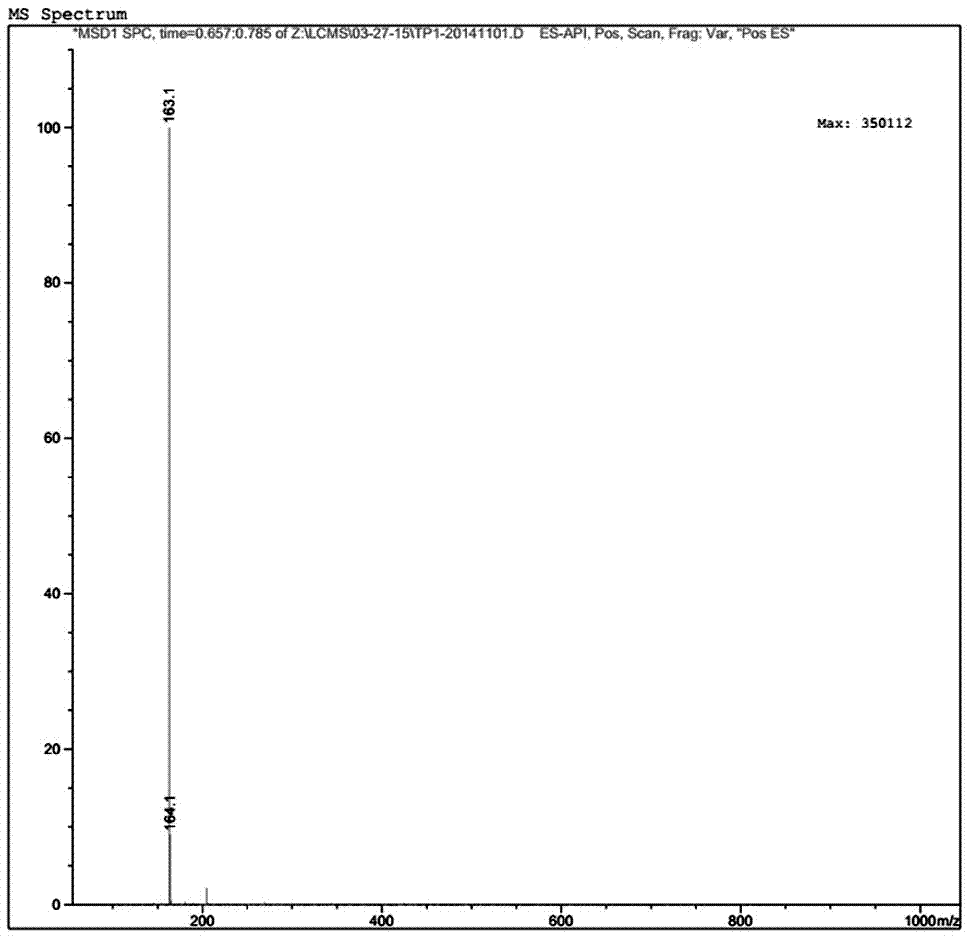
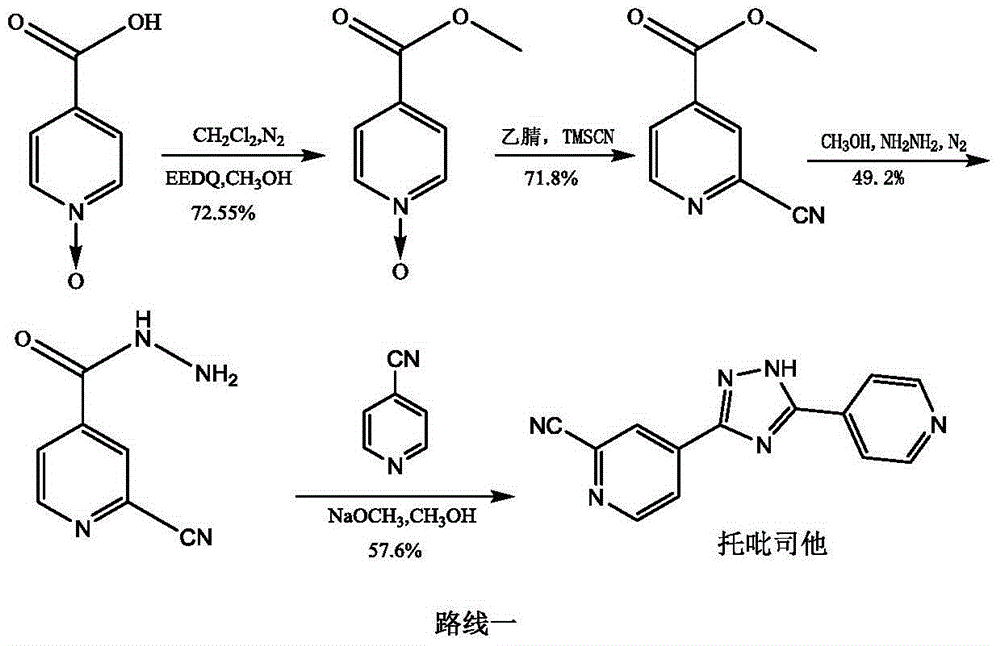
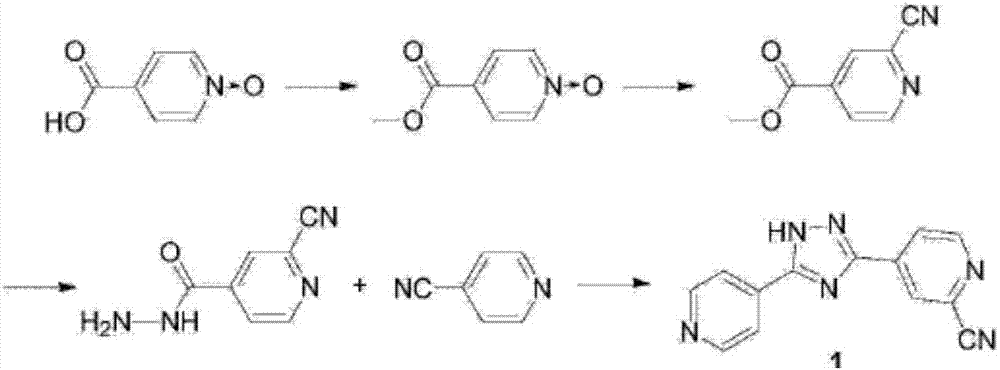
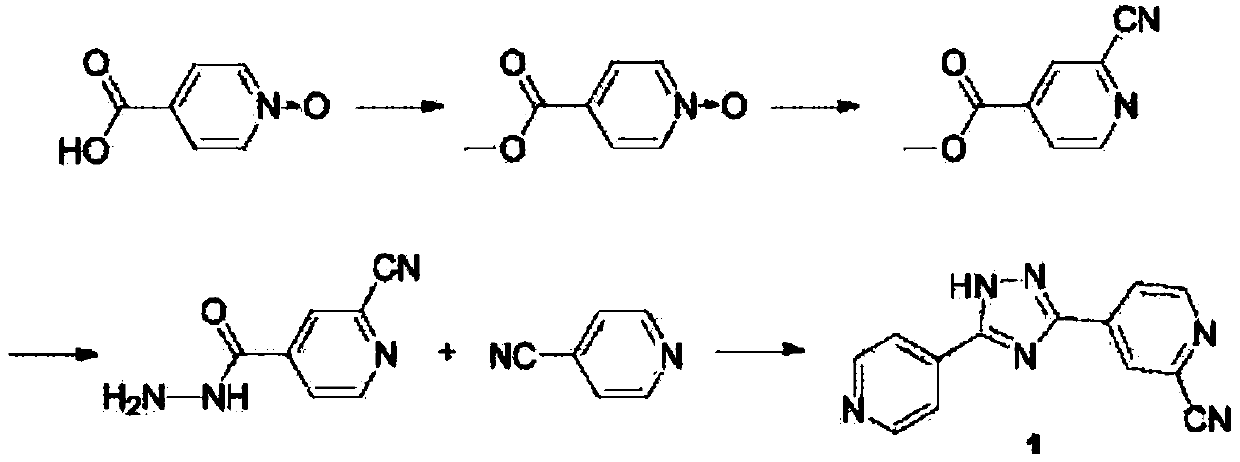
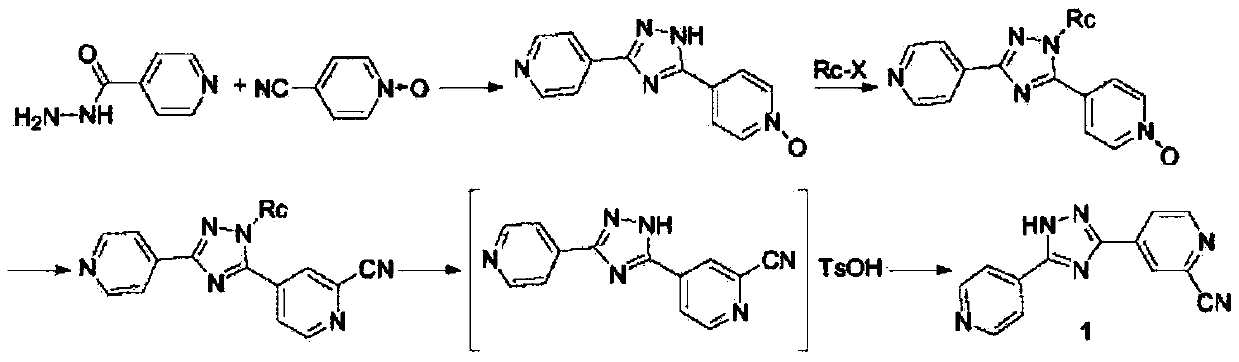
The invention relates to a preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile. The method includes following steps: preparing a compound methyl 2-cyanoisonicotinate represented as the formula (4) with a compound methylisonicotinic-N-oxide (5) being a starting raw material in the presence of a copper catalyst (CuX), a metal cyanide and dimethylcarbamyl chloride; performing hydrazinolysis to the methyl 2-cyanoisonicotinate to obtain a compound 2-cyanoisoniazide represented as the formula (3); and finally performing condensation with a compound 4-cyanopyridine represented as the formula (2) to obtain a compound topiroxostat represented as the formula (1). The method only comprises three steps and is simple in operation and post-treatment. By means of the copper catalyst, a usage amount of the metal cyanide is greatly reduced so that reaction conditions are milder. The method is high in purity of the prepared product and is suitable for industrial production.
Owner:王庆本 +1
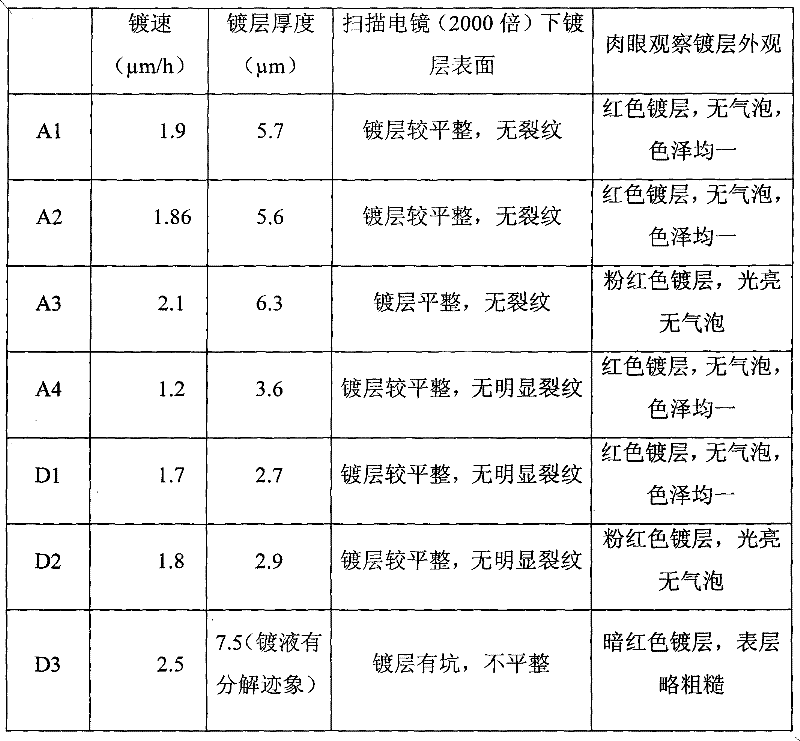
Chemical copper-plating solution and chemical copper-plating method
ActiveCN102534583AIncrease the maximum thicknessLiquid/solution decomposition chemical coatingNickel saltCopper plating
The invention provides chemical copper-plating solution and a chemical copper-plating method. The chemical copper-plating solution comprises copper salt, nickel salt, sodium monophosphate, a complexing agent, a stabilizing agent and water solution of a potential of hydrogen (pH) conditioning agent. The stabilizing agent contains 2-aminopyridine and 4 cyanopyridine. The complexing agent comprises triethanolamine and citrate, the content ratio of the triethanolamine to the citrate is 1:1-5:1, and the pH value of the chemical copper-plating solution is 8-11. By adopting the chemical copper-plating solution with the sodium monophosphate serving as a reducing agent, the thickness of a plating layer can reach 3-7 micrometers, so that the largest thickness of a traditional technology is greatly improved.
Owner:BYD CO LTD
Preparation technology for 5-(2-cyano4-pyridyl)-3-(4-pyridyl)-1,2,4-troazole
InactiveCN105130958ARelieve pressureSimple processOrganic chemistryHydrazine compoundOne-pot synthesis
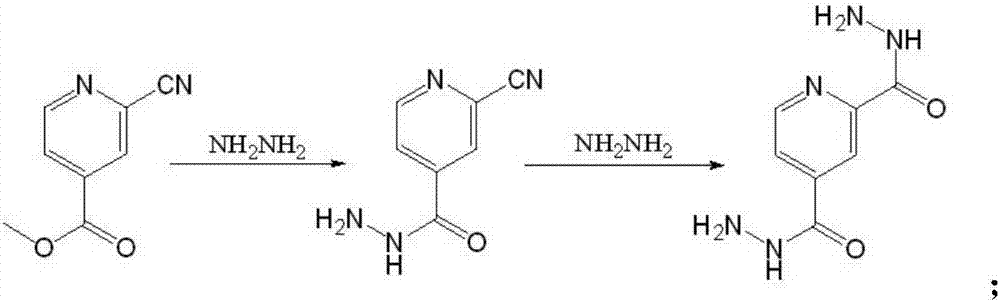
The invention belongs to the field of medicine and chemical engineering, and particularly discloses a preparation technology for 5-(2-cyano4-pyridyl)-3-(4-pyridyl)-1,2,4-troazole (topiroxostat). The preparation technology is characterized in that methyl isonicotinate serving as a compound II is used as a starting raw material, the compound II, phosphorus oxychloride, N,N-dimethyl formamide, iodine and 28% ammonium hydroxide form a compound III which is 2-cyano methyl isonicotinate in a one-pot synthesis mode, then a compound IV which is 2-cyano isonicotinyi hydrazine is formed through synthesis, a topiroxostat crude product Ia is prepared through condensing the compound IV which is 2-cyano isonicotinyi hydrazine and 4-cyanopyridine, salifying is performed on the crude product Ia and para-toluenesulfonic acid to form topiroxostat tosilate Ib, and finally the topiroxostat finished product I is obtained through desalting in a refined mode. The number of reactions steps is reduced, the yield of the finished product is increased, the reaction process is low in toxin and has small influences on environment, the reaction route is short, the cost is greatly lowered, the product purity is larger than 99.7%, the net contamination is smaller than 0.1%, and standard requirements are met.
Owner:JINAN KANGHE MEDICAL TECH
Synthesis method of 4-cyanopyridine
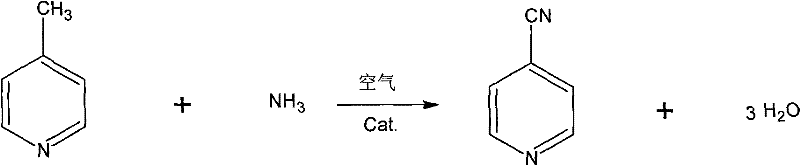
ActiveCN101602719ASimple processHigh yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalysts4-MethylpyridineSynthesis methods
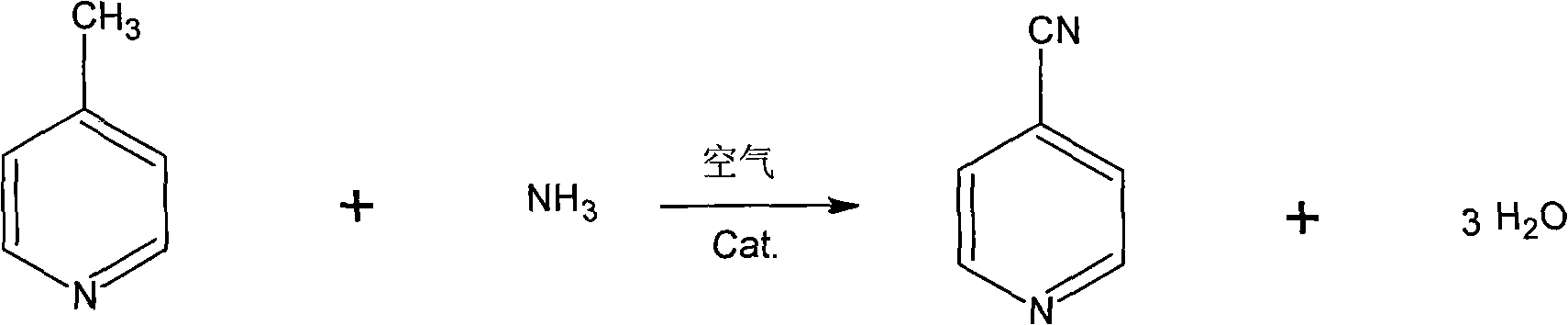
The invention discloses a synthesis method of 4-cyanopyridine, which is characterized in that 4-methylpyridine is vaporized and mixed with ammonia and air; the 4-methylpyridine reacts with the ammonia and air in the presence of a catalyst, and then the finished product of 4-cyanopyridine is obtained after absorption, extraction and rectification. The method features simple process and easy operation; the conversion rate of the 4-methylpyridine is more than 99% and the yield of the 4-cyanopyridine is more than 98%.
Owner:NANTONG ACETIC ACID CHEM
Preparation method for topiroxostat
The invention belongs to the field of medicine and chemical engineering, and particularly relates to a preparation method for topiroxostat. 2-chloro-4-[(5-pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]-pyridine is subjected to a cyanation reaction under the action of a cyanation reagent in the presence of a catalyst, base and a ligand to obtain topiroxostat. The preparation method comprises the following steps: 4-cyanopyridine-N-oxide is taken as a starting material, 1,2-dichloroethane is taken as a solvent, triethylamine is taken as base, phosphorus oxychloride is used as a chlorinated reagent, and chlorination is conducted to obtain 2-chloro-4-cyanopyridine; 2-chloro-4-cyanopyridine and isoniazide are in a methanol solvent, sodium methoxide is taken as a catalyst, and close-loop condensation is performed to obtain 2-chloro-4-[(5-pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]-pyridine. The preparation method has the advantages that a safe and cheap cyanogroup source is selected, a hypertoxic cyanation reagent is avoided, the environmental harm is reduced, the product yield is high, the purity is high, and the suitability for industrial mass production is high.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Efficient preparation of 4-dimethylaminopyridine
The invention discloses a new method for preparing 4-dimethylaminopyridine (DMAP) with a one-kettle process by taking 4-cyanopyridine and acrylic acid as main raw materials. The method mainly comprises the following steps: performing quaternization of 4-cyanopyridine with acrylic acid to obtain an intermediate; reacting with an amination reagent; neutralizing acid in the system in the presence of a pH regulator to enable the product to be free, wherein the reaction period is greatly shortened; and performing simple separation and purification to obtain a target product DMAP. In the method disclosed by the invention, the reaction conditions are mild, the synthesis method is simple, the DMAP synthesis can be efficiently performed through simple technological operation, and the obtained product has high purity and high yield; and the method is suitable for large-scale industrial production.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Preparation method of topiroxostat
ActiveCN107573330AReaction raw materials are readily availableThe reaction conditions are mild and easy to controlOrganic chemistryLoop closingTopiroxostat
The invention provides a preparation method of topiroxostat. The preparation method comprises the steps that 2-cyano methyl isonicotinate is used as raw materials; hydrazinolysis is performed at -10 DEG C to -20 DEG C to obtain an intermediate; the intermediate and 4-cyanopyridine react under the conditions with sodium ethoxide and the pH being 4 to 6 to obtain the topiroxostat. The preparation method has the advantages that the 2-cyano methyl isonicotinate is used as a starting material; the 2-cyano methyl isonicotinate and hydrazine hydrate take condensation reaction at low temperature to prepare the intermediate; the intermediate and the 4-cyanopyridine are subjected to condensation and loop closing under the acid condition with sodium ethoxide to prepare the topiroxostat. The raw materials can be easily obtained; the reaction conditions are mild and are easy to control; a reagent with high toxicity is not used in the reaction process; the released toxic substances are few; the sidereaction products are few; the reaction safety is high; the pollution is small; the obtained purity is high; the preparation method is suitable for industrial production.
Owner:HEBEI UNIV OF CHINESE MEDICINE +1
Preparation method of 4-pyridylaldehyde
InactiveCN102617454AReduced activityAvoid it happening againOrganic chemistryAcid waterHydrogenation reaction
The invention discloses a preparation method of 4-pyridylaldehyde, which belongs to the field of heterocyclic compounds, and comprises the following steps of: (1) adding 4-cyanopyridine into protonic acid water solution, adding a Cu-Ni catalyst, mixing uniformly, and adding hydrogen to carry out hydrogenation reaction until finishing the reaction; (2) filtering; (3) adding a neutralizing agent into the obtained 4-pyridylaldehyde acid solution filtered through the step (2), and neutralizing until a pH value reaches 4-6; and (4) separating and purifying the 4-pyridylaldehyde. According to the method, the Cu-Ni catalyst is adopted to carry out catalytic hydrogenation, so that the generation of 4-pyridinemethylamine, 4-pyridinemethanol and other impurities is avoided, the reaction period is short, and the yield and the purity are high.
Owner:CANGZHOU SENARY CHEM SCI TEC
Myricetin pharmaceutical eutectic crystal and preparation method thereof
ActiveCN103819440AImprove solubilityHigh dissolution rateOrganic chemistryHydrogenDrugs preparations
The invention discloses a myricetin pharmaceutical eutectic crystal and a preparation method thereof. The myricetin pharmaceutical eutectic crystal uses myricetin as the active pharmaceutical ingredients, uses theine, nicotinamide, pyrazinamide or 4-cyanopyridine as the precursors, and is a myricetin-theine eutectic crystal, a myricetin-nicotinamide eutectic crystal, a myricetin-pyrazinamide eutectic crystal or a myricetin-4-cyanopyridine eutectic crystal formed through intermolecular hydrogen bonds. The eutectic crystals are prepared by adopting the solution mediating crystal transformation method. The myricetin pharmaceutical eutectic crystal provided by the invention inherits the pharmacological activity of the myricetin; meanwhile, the solubleness, the dissolution rate and the stability of the myricetin pharmaceutical eutectic crystal are all significantly improved as compared with those of the myricetin; the myricetin pharmaceutical eutectic crystal facilitates the development of pharmaceutic preparation and the wide applications of the myricetin in the field of medicine.
Owner:SHANGHAI UNIV OF T C M
Topiroxostat and preparation method of intermediate of topiroxostat
InactiveCN108101840ARaw materials are cheap and easy to getThe reaction is easy to operateOrganic chemistryNitrogenTopiroxostat
4-cyanopyridine is taken as a raw material, a compound III is obtained through a reaction, the compound III and a compound IIB undergo an amidation reaction to obtain a compound IV, and a ring-closurereaction is carried out in the function of an acid catalyst to obtain a target topiroxostat molecule. According to the scheme, the raw materials are cheap and are easy to obtain, the product qualityis high, the reaction is simple to operate and is mild, the yield is high, and the waste gas, waste water and industrial residue are less produced. In the reaction process, nitrogen protection isn't required, and a cyaniding reagent isn't used, so that the method has bright industrial prospects.
Owner:NANJING HUAWE MEDICINE TECH DEV
High performance liquid chromatographic detection method for 3-cyanopyridine and 4-methylpyridine in 4-cyanopyridine
The invention discloses a high performance liquid chromatographic detection method for 3-cyanopyridine and 4-methylpyridine in 4-cyanopyridine. The high performance liquid chromatographic detection method comprises the following steps: (1) preparing a system applicable solution; (2) preparing a test solution; 3) preparing a contrast solution. The system applicable solution, the test solution and the contrast solution are detected respectively by adopting high performance liquid chromatography. The detection condition is as follows: octadecylsilane chemically bonded silica is used as a filling agent of a chromatographic column; a mobile phase A is modified buffer salt, and is prepared by adding 20 mmol of potassium dihydrogen phosphate and 2.4 ml of triethylamine into 1000 ml of water and adjusting the pH value to 6.0-8.0 with phosphoric acid; a mobile phase B is an organic phase, and adopts gradient elution; the flow rate is 0.5-1.5 ml / min; the column temperature is 10-40 DEG C; the detection wave length is 200-300 nm; the injected sample volume is 10 [mu]l. The separation degree of 3-cyanopyridine, 4-cyanopyridine and 4-methylpyridine in the detection method are all 2.0 or above, the theoretical plate number is high, the symmetry is good, and the condition that the accuracy of the detection results is influenced by interference between the components is effectively avoided; meanwhile, the detection method has the advantages of being accurate and reliable in detection result, short in analysis time, simple and convenient to operate, and the like.
Owner:江苏悦兴医药技术有限公司
Topiroxostat preparation technology
ActiveCN105566301AImprove solubilityIncreased rate of release of cyanide ionsOrganic chemistryPotassium ferrocyanideHydrazide
The invention discloses a topiroxostat preparation method. According to the method, 2-chloroisonicotinic acid which is cheap and easy to obtain serves as an initial material, potassium ferrocyanide serves as a green cyanogens source, AgI-KI-PEG generates 2-cyanoisonicotinate through cyanation of a mixed catalysis system and then directly acts with hydrazine hydrate to obtain 2-cyanoisonicotinate hydrazide under the action of an amide condensing agent phenyl dichlorophosphate (PDCP), and the product and 4-cyanopyridine are condensed to obtain topiroxostat. Through technological improvement of the reaction, reaction steps are shortened, the reaction yield is obviously increased by 90% or above, and the technological cost is obviously reduced. Meanwhile, a 2-chloroisonicotinic acid raw material which is cheaper and easy to obtain is used, use of corrosive thionyl chloride and other chlorinating agents is avoided, and the technology is suitable for industrial production. The method is short in synthesis step, easy to operate, mild in reaction condition, economical, environmentally friendly and suitable for industrial production, and the yield is obviously increased.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Mixed ligand-based zinc complex and preparation method thereof
InactiveCN103864825AStrong purple luminescent propertiesPolycrystalline material growthLuminescent compositionsTetrazoleSodium azide
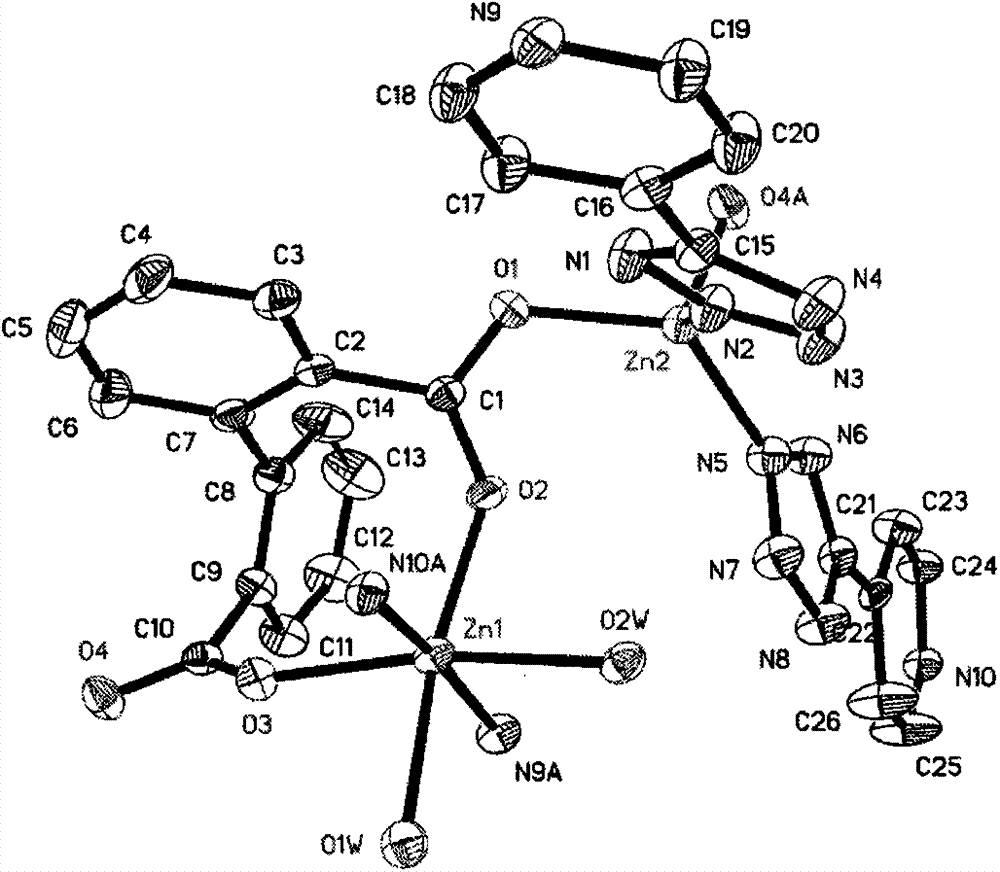
The invention discloses a mixed ligand-based zinc (II) complex and a preparation method thereof. The method comprises the following step: preparing colorless lump crystal from zinc nitrate hexahydrate, sodium azide, 4-cyanopyridine and 2,2'-diphenyldicarboxylic acid in secondary deionized water by hydrothermal in situ reaction, wherein the molar ratio of zinc nitrate hexahydrate to sodium azide to 4-cyanopyridine to 2,2'-diphenyldicarboxylic acid is 2:2:2:1; the reaction medium is secondary deionized water; the specific steps are as follows: reacting at constant temperature of 180 DEG C for 3 days, then cooling to room temperature, filtering, washing and drying to obtain the colorless lump crystal; the chemical formula is Zn<2>(PTZ)<2>(DPA)(H<2>O)<2>; PTZ is 5-(4-pyridine) tetrazole monovalent anion obtained from sodium azide and 4-cyanopyridine by [2+3] in situ reaction under the hydrothermal condition; DPA is divalent anion of 2,2'-diphenyldicarboxylic acid. The complex belongs to a functional coordination complex, has a novel two-dimensional layer structure, is good in thermal stability, and can be further developed and applied as a molecular-based light-emitting material. The preparation method disclosed by the invention is simple and available in raw material.
Owner:ANQING NORMAL UNIV
Method for cooling, crystallizing and separating 4-cyanopyridine by solvent
InactiveCN108997211ASolving Recycling ProblemsReduce energy consumptionOrganic chemistryEconomic benefitsSlurry
The invention discloses a method for cooling, crystallizing and separating 4-cyanopyridine by a solvent. The method comprises the following steps: (1) performing decoloring treatment on a mixed cyanopyridine raw material containing 3-cyanopyridine and 4-cyanopyridine at high temperature; (2) dissolving the mixed cyanopyridine raw material after decoloring treatment into a preponderant solvent methanol in a crystallizer and preparing a supersaturated solution; and (3) cooling the supersaturated solution at a certain cooling rate to final temperature to separate out the 4-cyanopyridine, discharging the 4-cyanopyridine and mixed cyanopyridine mother liquid out of the crystallizer in a form of slurry, entering a filter, separating the 4-cyanopyridine and the mixed cyanopyridine mother liquid in the filter and drying to obtain a high-purity 4-cyanopyridine product. The method has the advantages of low energy consumption and low cost through cooling, crystallization and separation; furthermore, one-step crystallization is only needed after the raw materials are subjected to decoloring treatment, and the product purity can reach to 99.2 to 99.7 percent. By the method, the production costis low and the economic benefit is good; meanwhile, the problem about recycling of waste is solved.
Owner:SUN YAT SEN UNIV
Preparation process and method for topiroxostat
InactiveCN105294656AGuaranteed homeostasis responseQuality improvementOrganic chemistryTopiroxostatReagent
The invention relates to a preparation process and method for topiroxostat. 4-cyanopyridine [formula (II)] and 2-cyanoisonicotinohydrazide [formula (III)] are subjected to a condensation reaction by stages to prepare [formula (IV), 4-pyridylcarbonylhydrazine-Nminute-(2-cyanopyridine-4- carbimide], washing the [formula (IV)] and then carrying out a cyclization reaction to prepare the topiroxostat [(formula (I)]. The preparation process disclosed by the invention is simple and convenient, free of special toxic reagent, green and environment-friendly, good in product yield, good in quality and in conformity with the medicinal requirement.
Owner:大道隆达(北京)医药科技发展有限公司
One-pot method for synthesizing Topiroxostat
The invention discloses a one-pot method for synthesizing Topiroxostat. The one-pot method comprises the following steps: dissolving 2-cyano methyl isonicotinate into a solvent, reacting with hydrazine hydrate to generate an intermediate 2-cyanoisoniazide, then adding alkali for reaction in the same reactor, then adding 4-cyanopyridine to form a ring, and finally purifying to obtain the Topiroxostat. The one-pot method is short in technological process, simple in operation, high in raw material utilization rate and low in production cost, and has higher production and practical value.
Owner:NANJING UNIV OF TECH
Production method of nicotinic acid
The invention discloses a production method of nicotinic acid. The production method comprises the following steps: adding 4-cyanopyridine and de-ionized water to a container, and stirring to obtain a liquid; heating and warming up, adding nitrilase and carrying out a reaction to obtain a nicotinic acid liquid; and decompressing and distilling to obtain a concentrated liquid, drying, tabletting and breaking the concentrated liquid to obtain nicotinic acid particles. According to the production method disclosed by the invention, the content of the nicotinic acid prepared by virtue of the nitrilase is above 95%, and meanwhile the production method is mild in reaction condition, stable in product, simple to operate and easy to implement. The production method disclosed by the invention is free from materials corrosive to equipment during preparation, so that the service life of the equipment is prolonged; and the production method is free from pollution to environment, and is especially suitable for large-scale industrial production.
Owner:XUZHOU HENGDING BIOTECH
Method for controlling impurities of isoniazid
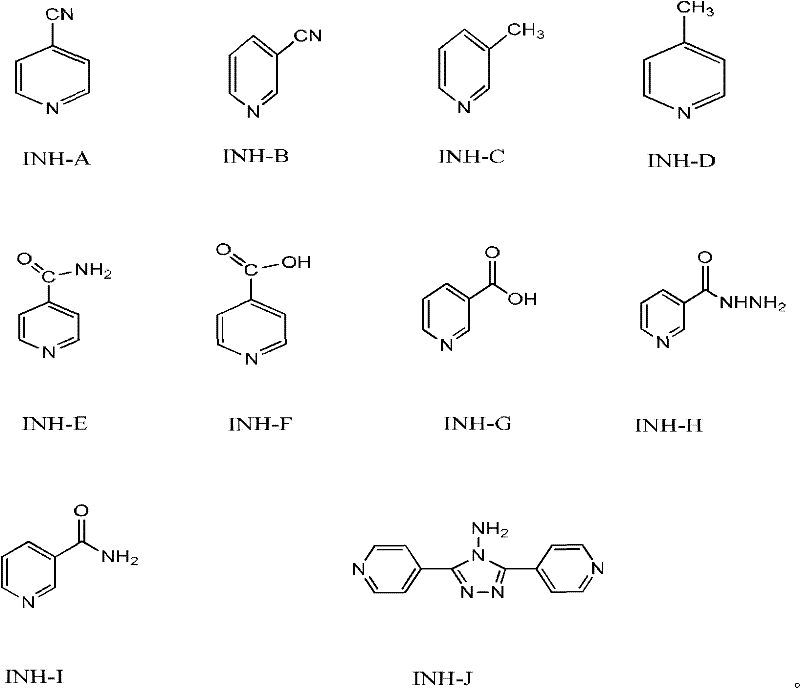
The invention discloses a method for controlling impurities of isoniazid, which comprises the following steps of: controlling the chromatographic purity of a 4-cyanopyridine material to be greater than or equal to 99.8%, wherein the content of an INH-C(Isonicotinyl Hydrazine-C) impurity is less than or equal to 0.1%, the content of an INH-D(Isonicotinyl Hydrazine-D) impurity is less than or equal to 0.1%, and the content of an INH-B(Isonicotinyl Hydrazine-B) impurity is less than or equal to 0.1%; in a hydrolytic reaction step, controlling the reaction temperature to be 95-105 DEG C and the reaction time to be 1.5-3 hours; in a condensation reaction step, controlling the reaction temperature to be 90-97 DEG C and the reaction time to be 1.5-2.5 hours; controlling the molar ratio of the 4-cyanopyridine to hydrazine hydrate to be 1:(2-4); and obtaining isoniazid with high purity and low impurity. The method disclosed by the invention is simple in operation, easy to control and suitable for industrialized operation.
Owner:浙江新赛科药业有限公司
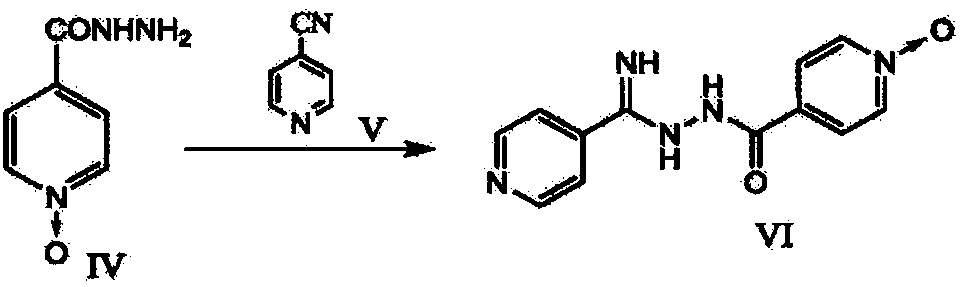
New topiroxostat synthesis intermediate and preparation method thereof
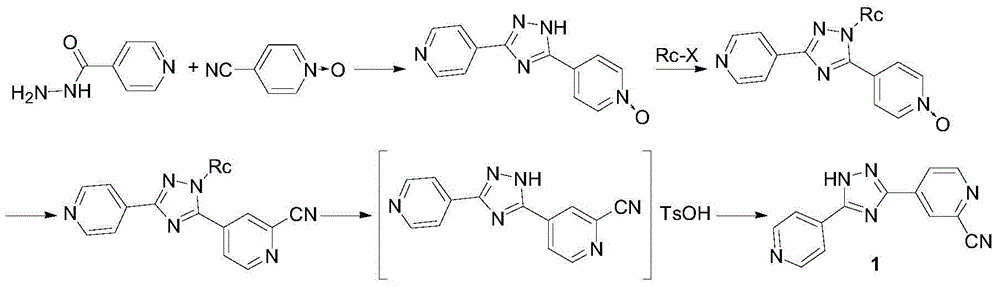
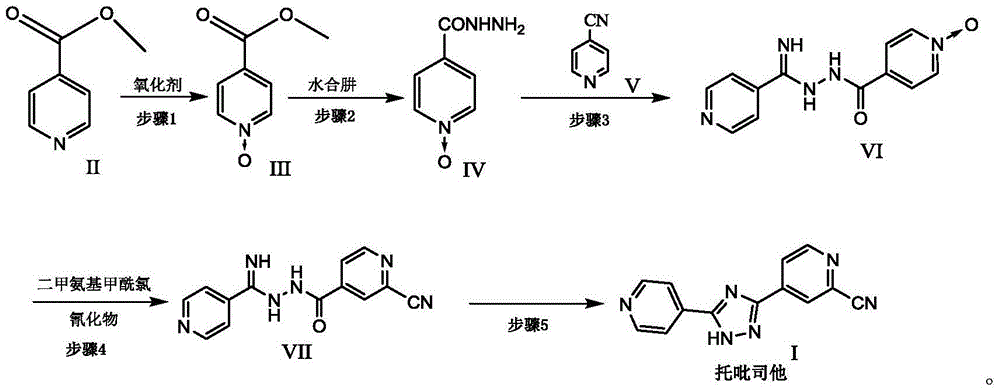
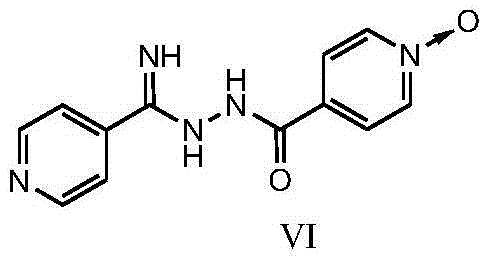
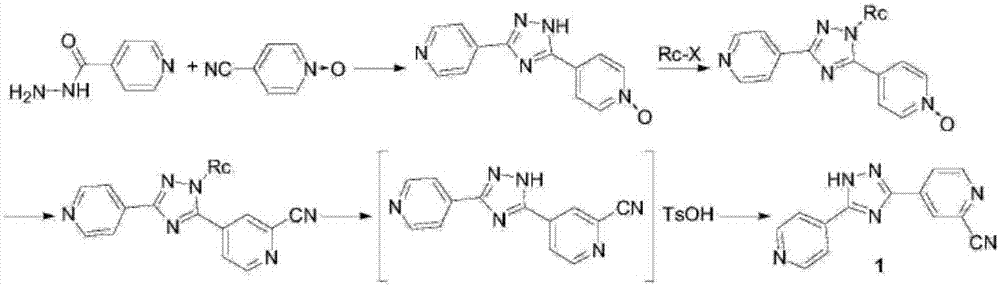
The present invention provides a new topiroxostat synthesis intermediate 4-(2-(imino(pyridine-4-yl)methyl)hydrazinocarbonyl)pyridine N-oxide (compound VI) and a preparation method thereof, wherein isoniazid N-oxide IV and 4-cyanopyridine V are subjected to a reaction in a suitable solvent under an alcohol alkali condition to obtain the product, the alcohol alkali is selected from sodium methoxide, sodium ethoxide, potassium ethoxide or potassium t-butoxide, and the reaction formula is defined in the specification. According to the present invention, through the compound VI, the gout treating drug topiroxostat can be prepared under the mild and easy industrial control reaction conditions.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Synthesis method of topiroxostat
The invention belongs to the field of medicine and chemical industry and discloses a synthesis method of topiroxostat. The method comprises that 4-cyanopyridine as a starting raw material is oxidized by hydrogen peroxide to form an intermediate 1, the intermediate 1, sodium methoxide and ammonium chloride undergo a reaction to produce an intermediate 2, the intermediate 2 and 4-cyanopyridine undergo an annulation reaction under action of cuprous bromide and sodium carbonate to produce an intermediate 3, and the intermediate 3 and trimethylsilyl cyanide undergo a cyanation reaction to produce topiroxostat. The method utilizes low-price 4-cyanopyridine as a starting raw material, and in the first reaction step, methanol is used as a solvent and 4-cyanopyridine nitrogen oxide as the intermediate 1 is prepared. The method has a high yield and produces a high-purity product. The whole route is easy to operate and is conducive to industrial mass production.
Owner:KAIFENG PHARMA GRP +2
A kind of synthetic method of topicastat
Owner:KAIFENG PHARMA GRP +2
Synthesis method of 2-(4-cyano) pyridyl (3-cyano) thioanisole
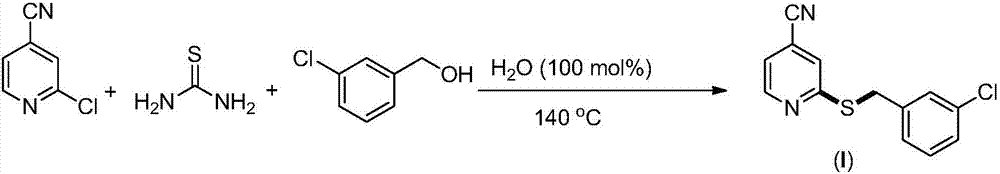
The invention provides a synthesis method of 2-(4-cyano) pyridyl (3-cyano) thioanisole. The invention also provides a synthesis method of 4-(2-formamido) pyridyl (3-bromo) thioanisole. According to the technical scheme, 2-chloro-4-cyanopyridine / 4-chloropyridine-2-formamide, micromolecular sulfur compounds and 3-chloro / bromobenzyl alcohol are used for directly synthesizing 2-(4-cyano) pyridyl (3-cyano) thioanisole (I) and 4-(2-formamido) pyridyl (3-bromo) thioanisole (II) under the condition of no additional catalysts. The method has the advantages that the reaction conditions are simple; inert gas protection is not needed; solvents are not needed; the operation is easy. By the method, the 20-time amplification production can be conveniently realized; the product gram grade preparation is performed, so that certain study and industrial application prospects are realized.
Owner:WENZHOU UNIVERSITY
Myricetin drug co-crystal and preparation method thereof
ActiveCN103819440BImprove solubilityHigh dissolution rateOrganic chemistryHydrogenDrugs preparations
The invention discloses a myricetin pharmaceutical eutectic crystal and a preparation method thereof. The myricetin pharmaceutical eutectic crystal uses myricetin as the active pharmaceutical ingredients, uses theine, nicotinamide, pyrazinamide or 4-cyanopyridine as the precursors, and is a myricetin-theine eutectic crystal, a myricetin-nicotinamide eutectic crystal, a myricetin-pyrazinamide eutectic crystal or a myricetin-4-cyanopyridine eutectic crystal formed through intermolecular hydrogen bonds. The eutectic crystals are prepared by adopting the solution mediating crystal transformation method. The myricetin pharmaceutical eutectic crystal provided by the invention inherits the pharmacological activity of the myricetin; meanwhile, the solubleness, the dissolution rate and the stability of the myricetin pharmaceutical eutectic crystal are all significantly improved as compared with those of the myricetin; the myricetin pharmaceutical eutectic crystal facilitates the development of pharmaceutic preparation and the wide applications of the myricetin in the field of medicine.
Owner:SHANGHAI UNIV OF T C M
Preparation process of 5-(2-cyano4-pyridyl)-3-(4-pyridyl)-1,2,4-triazole
The invention belongs to the field of medicine and chemical industry, and specifically discloses a preparation of 5-(2-cyano4-pyridyl)-3-(4-pyridyl)-1,2,4-triazole (topinostat) The process is characterized in that the compound III 2-cyano group is synthesized by a one-pot method using compound II methyl isonicotinate as a starting material and phosphorus oxychloride, N,N-dimethylformamide, iodine simple substance, and 28% ammonia water Methyl isonicotinate, then compound IV 2-cyanoisonicotinic acid hydrazide is synthesized, and then compound IV2-cyanoisonicotinic acid hydrazide is condensed with 4-cyanopyridine to prepare topinostat crude product Ia, and crude product Ia is passed through with p-toluene After the sulfonic acid is salified, topinastat p-toluenesulfonate Ib is formed, and finally desalted and refined to obtain the finished product I of topinostat. The invention reduces the reaction steps, improves the yield of finished products, has low toxicity in the reaction process, has little impact on the environment, has a short reaction route, and greatly reduces the cost.
Owner:JINAN KANGHE MEDICAL TECH
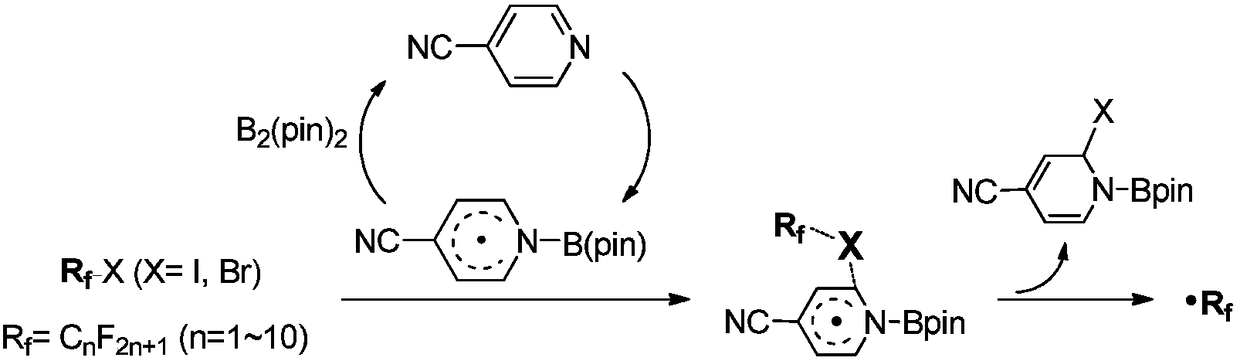
Activation method of perfluoroalkyl carbonhalogen bond and method for synthesizing pyridine derivative
The invention discloses an activation method of a perfluoroalkyl carbon-halogen bond and a method for synthesizing a pyridine derivative. The activation method is characterized by comprising the stepthat: active pyridine-boron free radicals generated by reaction between 4-cyanopyridine and pinacol biborate are used for catalyzing the homolysis of a carbon-halogen bond of perfluoroalkyl halide toform perfluoroalkyl free radicals. Perfluoroalkyl of olefin and a pyridine difunctionalized pyridine derivatization product are obtained by reaction by taking the pinacol biborate, the perfluoroalkylhalide (or bromide), the 4-cyanopyridine and the olefin as initiators. The method for synthesizing the pyridine derivative, provided by the invention, can simultaneously introduce two important groups, namely the perfluoroalkyl and the pyridine into the olefin for the synthesis of the pyridine derivative, thereby having the value of developing a novel method for the industrial synthesis of a pyridine compound.
Owner:NANJING UNIV
Caesium adsorbing material based on sodium modified zeolite and preparation method thereof
InactiveCN107855111AEnvironmentally friendlyImprove adsorption efficiencyOther chemical processesWater contaminantsPyridazinePyrazine
The invention discloses a caesium adsorbing material based on sodium modified zeolite. The caesium adsorbing material is prepared by the following steps of cleaning zeolite, and modifying by a mixed liquor which is prepared from NaCl (sodium chloride), ammonium hydroxide, diisopropylbenzene diphenylamine, FeCl3 (ferric trichloride) and FeCl2 (ferrous chloride), so as to prepare a matter A04; modifying the matter A04 by a mixed liquor which is prepared from 2-bromomethyl thiophene, 4-methyl-5-aminopyrimidine, 6-propoxypyridazine-3-amine and 2-amino-6-chloro-3-formylchromone, so as to prepare asubstance A; modifying the substance A by a mixed liquor which is prepared from ZrCl4 (zirconium tetrachloride), InCl3 (indium chloride), Zn(NO3)2 (zinc nitrate) and Sr(NO3)2 (strontium nitrate), so as to prepare a substance B; modifying the substance B by a mixed liquor which is prepared from 3-amino-4-cyanopyridine, diphenylcarbazide and 2-methyl-3-(methylmercapto) pyrazine, so as to prepare a substance C; modifying the substance C by a mixed liquor which is prepared from 2-chloro-3-aminopyrazine, 3-amino-5-bromine-2-fluoropyrimidine and 3-amino-6-(propylthio)pyridazine, so as to obtain thecaesium adsorbing material based on the sodium modified zeolite.
Owner:北京源清益壤环保科技有限公司
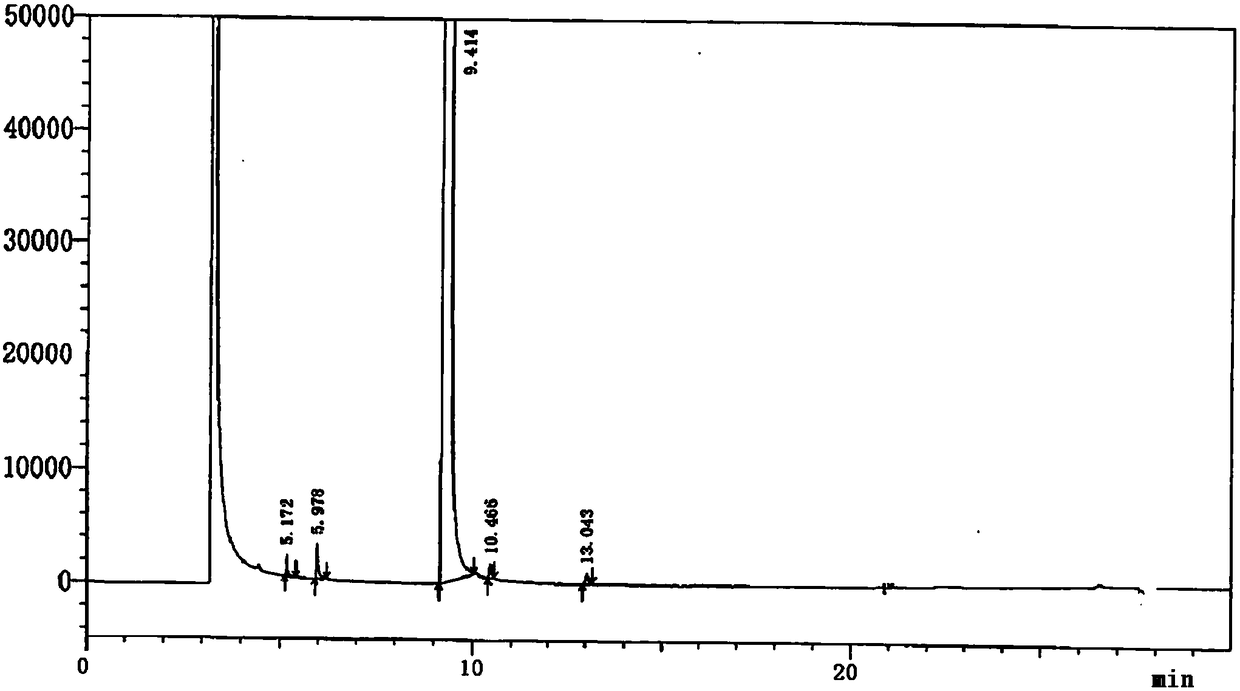
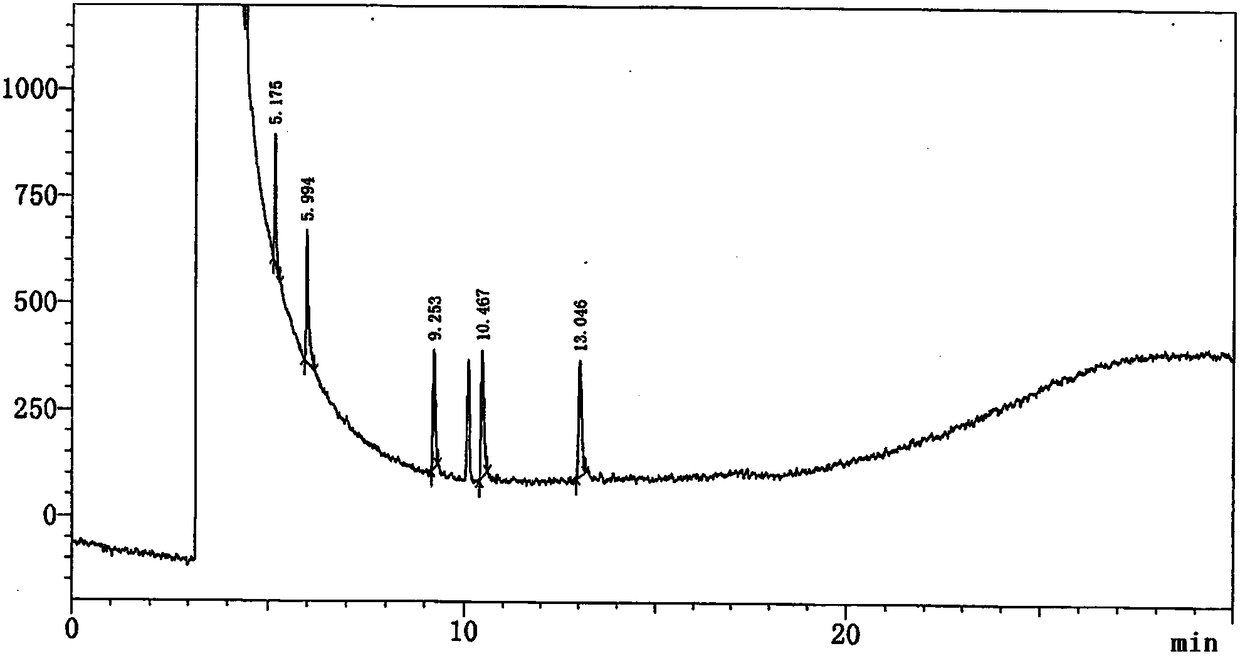
Method for determining content of 4-cyanopyridine and impurities of 4-cyanopyridine in isoniazide starting material
InactiveCN108226323AEfficient separationDo not interfere with detectionComponent separationProduct gasVaporization
The invention belongs to the field of analytical chemistry, and particularly relates to a method for determining the content of 4-cyanopyridine and impurities of 4-cyanopyridine in an isoniazide starting material. The measuring method is a gas chromatographic method, and comprises the following specific steps: vaporizing a sample in a vaporization chamber, and introducing the vaporized sample intoa chromatographic column through flowing gas; separating the 4-cyanopyridine SM1 and the impurities of 4-cyanopyridine by means of programmed heating; calculating the content of the impurities by using a principal component self-contrasted method with a correction factor. At present, no reports of simultaneous separation and determination of a plurality of impurities in the SM1 exist. The methodfor measuring the content of the plurality of impurities in the SM1 provided by the invention is simple and feasible, and has high sensitivity and high specificity, so that established quality controlitems of SM1 relevant substances can improve the quality controllability and safety of a product when being applied to control of relevant substances of the product.
Owner:CHONGQING HUABANGSHENGKAI PHARM
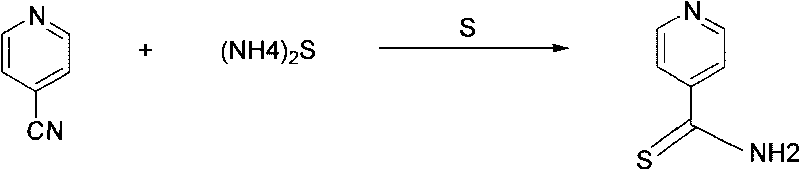
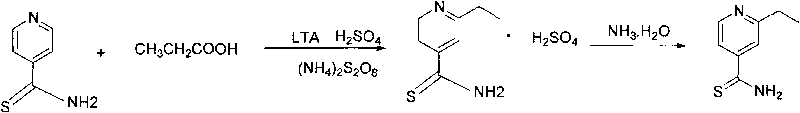
Preparation method of 2-alkyl thioisonicotinamide
ActiveCN101723892AHighlight substantive featuresSignificant progressOrganic chemistryAmmonium sulfideFatty acid
The invention provides a preparation method of 2-alkyl thioisonicotinamide, comprising the following steps of: preparing thioisonicotinamide through the reaction between 4-cyanopyridine as raw material and ammonium sulfide under the catalysis of sulphur; preparing crude 2-alkyl thioisonicotinamide through the hydrocarbonylation reaction between the thioisonicotinamide and fatty acid under the catalysis of lead tetraacetate (LTA), and refining to obtain the fine product by adopting ethanol. By adopting the reaction route of first sulfuric ammonization and then hydrocarbonylation, the positioning effect of the hydrocarbonylation reaction is improved, the generation of isomerized products in the reaction is reduced, and the operation is simplified; by adopting lead tetraacetate to substitute high-price silver nitrate, not only the cost is reduced, but also the reaction conversion ratio is improved, the two-step yield reaches up to 64 percent, the product purity reaches up to more than 98.5 percent, and the scale production is easy to realize.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Synthesis method of 4-cyanopyridine
ActiveCN101602719BSimple processHigh yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalysts4-MethylpyridineSynthesis methods
Owner:NANTONG ACETIC ACID CHEM
Pseudomonas putida and method for producing nicotinic acid or isonicotinic acid through converting Pseudomonas putida
ActiveCN102337233BReduce manufacturing costSuitable for mass productionBacteriaMicroorganism based processesPseudomonas putidaNitrilase activity
The invention discloses a new bacterial strain CGMCC3830 (Pseudomonas putida) and a method for catalytically producing nicotinic acid or isonicotinic acid by using the bacterial strain. In the invention, the nicotinic acid or isonicotinic acid is obtained through a hydrolysis reaction, wherein 3-cyanopyridine and 4-cyanopyridine are taken as raw materials, and the CGMCC3830 (Pseudomonas putida) with high nitrilase activity obtained through fermentation culture is taken as a catalyst. The method comprises the following steps of: adding a substrate in a reactor in a flowing mode, and reacting for 6-10 hours to obtain 147g / L-221g / L of nicotinic acid or isonicotinic acid. The CGMCC3830 (Pseudomonas putida) has the characteristics of short life cycle, strong vital force, high conversion efficiency, short conversion cycle and the like, the production cost of nicotinic acid or isonicotinic acid is reduced greatly. The production process has the characteristics of mild reaction conditions, low energy consumption and high yield, is environmentally-friendly and the like.
Owner:JIANGNAN UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile](https://images-eureka.patsnap.com/patent_img/55e48882-21a1-424c-b066-a4def80b3d6e/BDA0000560558790000021.PNG)
![Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile](https://images-eureka.patsnap.com/patent_img/55e48882-21a1-424c-b066-a4def80b3d6e/BDA0000560558790000022.PNG)
![Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile](https://images-eureka.patsnap.com/patent_img/55e48882-21a1-424c-b066-a4def80b3d6e/BDA0000560558790000023.PNG)