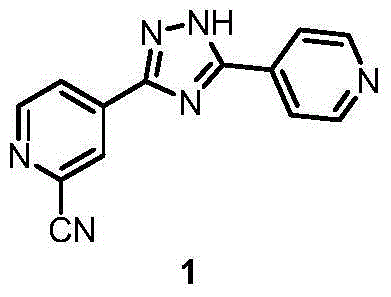
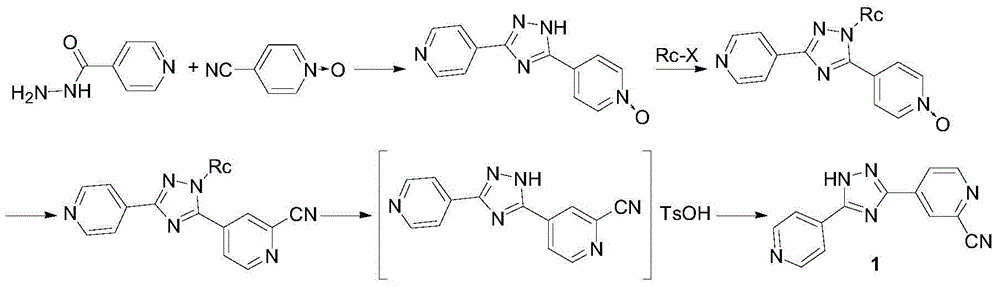
Preparation method for topiroxostat
A technology of topiramate and pyridine, applied in the field of preparation of topirastat, can solve the problems of high difficulty in industrialization, low yield and the like, and achieve the effects of reducing operation difficulty, high yield, and reducing post-reaction processing burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
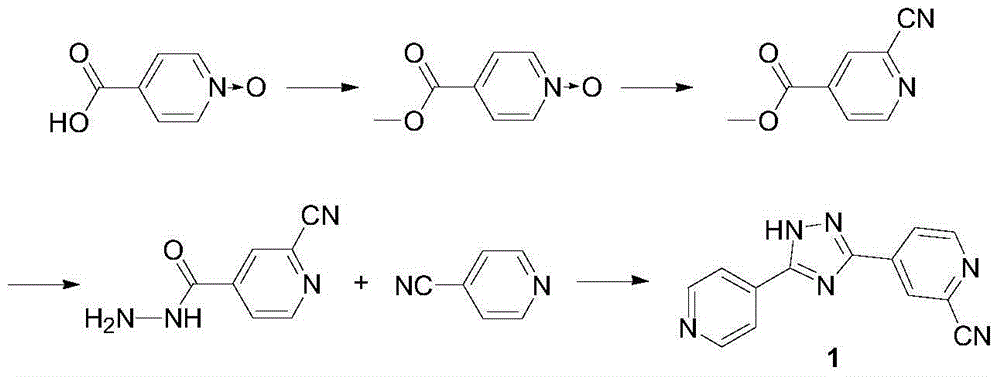
[0035] The preparation method of the compound of formula 5 used in embodiment 1-4 is:
[0036] In a 1000ml reaction flask, add 120g of 4-cyanopyridine-N-oxide, 360ml of 1,2-dichloroethane, 183.6g of phosphorus oxychloride, cool the reaction system to -2±2°C, add three Ethylamine 151.5g, dropwise time is 2h, after the dropwise addition is completed, keep warm for 2h, after the reaction is completed, the reaction solution is concentrated until no more fractions flow out, add 240ml of water, stir, a large amount of solids are precipitated, suction filtration, filter cake Rinse with water and dry to obtain 117.5 g of off-white solid, namely 2-chloro-4-cyanopyridine.
[0037] In a 1000ml reaction flask, add 110g of 2-chloro-4-cyanopyridine, add 660ml of methanol, 0.86g of sodium methoxide, stir at 25°C for 2h, then add 120g of isoniazid, continue to stir at 25°C for 2h, then Raise the temperature to methanol reflux, keep it warm for 12 hours, cool the reaction solution to room tem...
Embodiment 1
[0039] In a 2500ml reaction flask, add 180g of compound of formula 5, 13.3g of catalyst CuI, 18.5g of ligand DMEDA, KI 60g of K 4 [Fe(CN) 6 ]128.65g, Na 2 CO 3 74g, N,N-dimethylformamide 1080ml, under the protection of nitrogen, heat up to 120°C, keep warm for 10h, cool to room temperature, filter with suction, rinse the filter cake with water, and dry to obtain a white to light yellow solid, namely the product Topirastat, the yield is 80%.
Embodiment 2
[0041] In a 2500ml reaction flask, add 180g of compound of formula 5, 33.0g of catalyst CuI, 92.5g of ligand DMEDA, KI 116g of K 4 [Fe(CN) 6 ]77.2g, Na 2 CO 3 59.2g, N-methylpyrrolidone 1080ml, under the protection of nitrogen, heat up to 150°C, keep warm for 24h, cool to room temperature, filter with suction, rinse the filter cake with water, and dry to obtain a white to light yellow solid, namely the product topiramate He, the yield was 75.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com