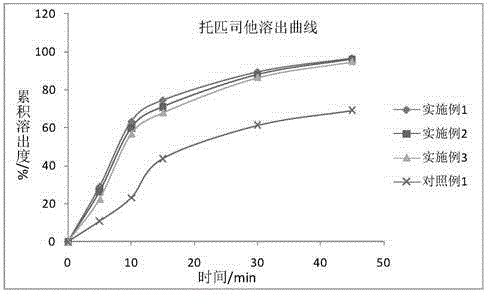
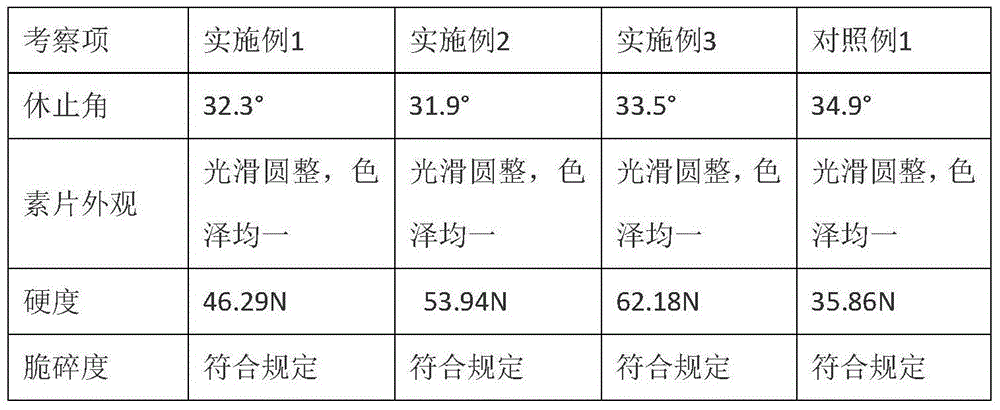
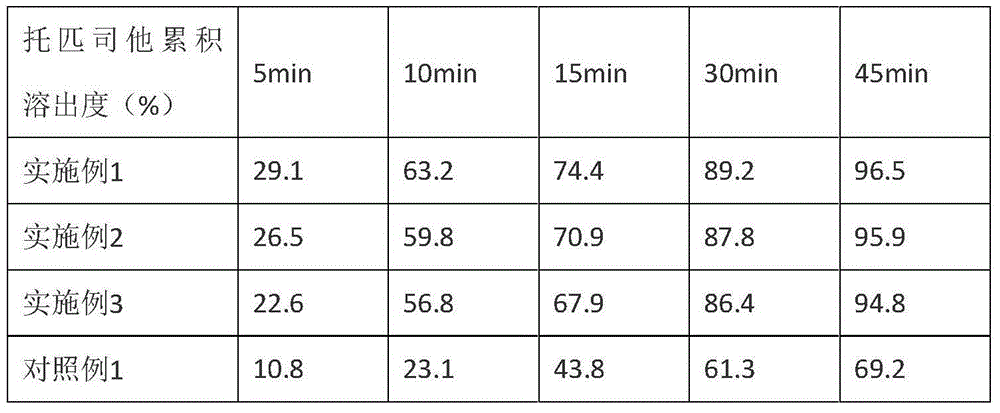
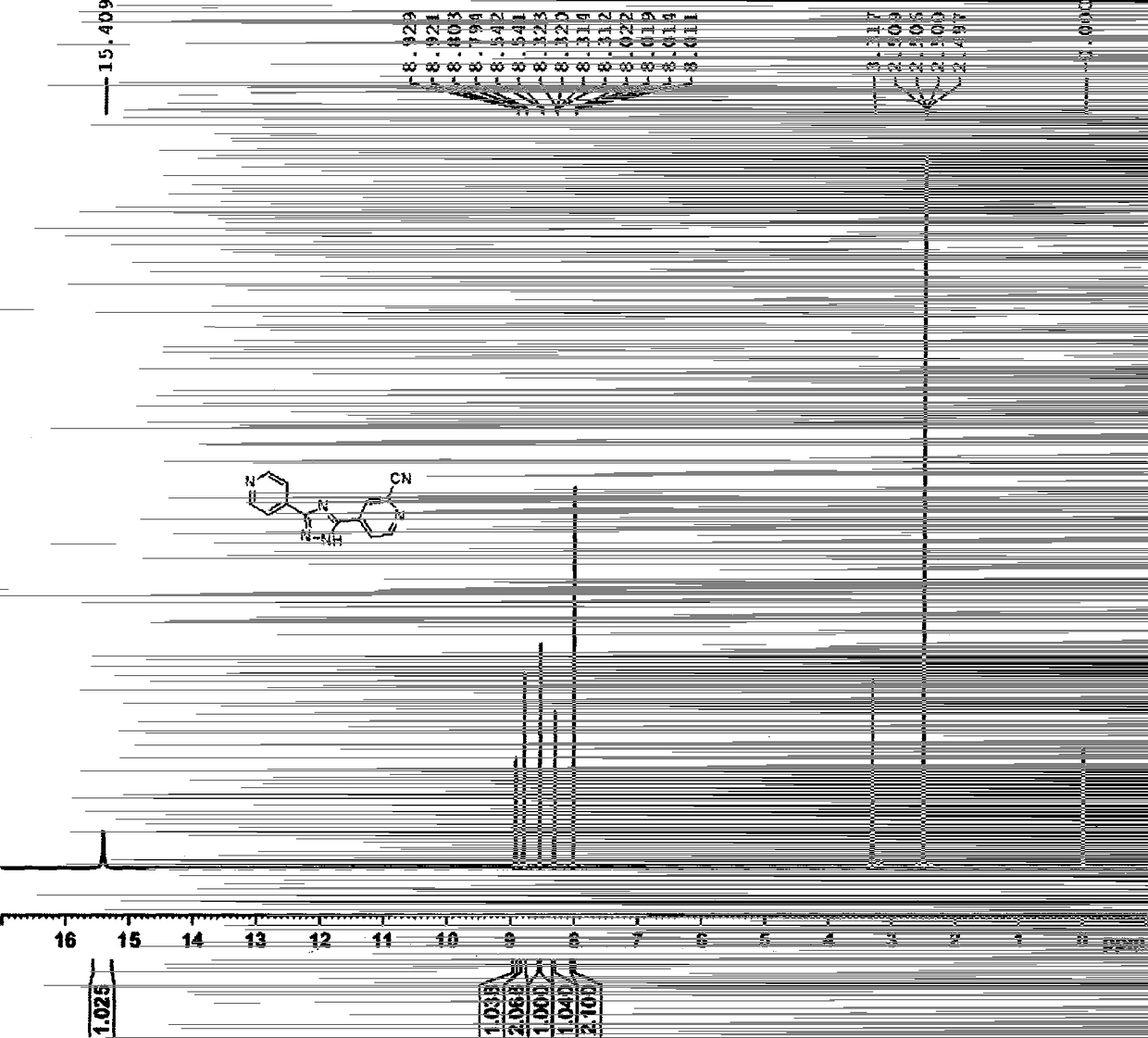
Patents
Literature
53 results about "Topiroxostat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
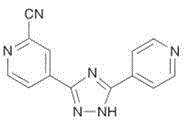
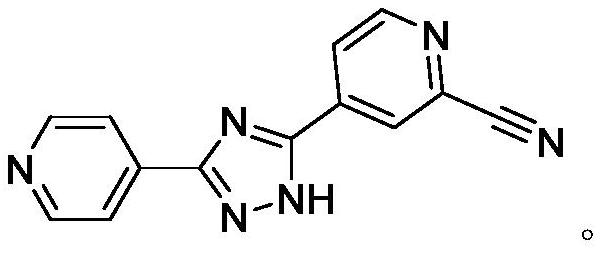
Topiroxostat (INN; trade names Topiloric, Uriadec) is a drug for the treatment of gout and hyperuricemia. It was approved for use in Japan in June 2013. Topiroxostat is a xanthine oxidase inhibitor which reduces serum urate levels.
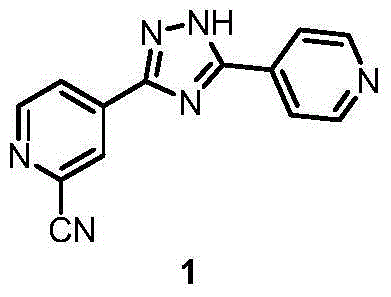
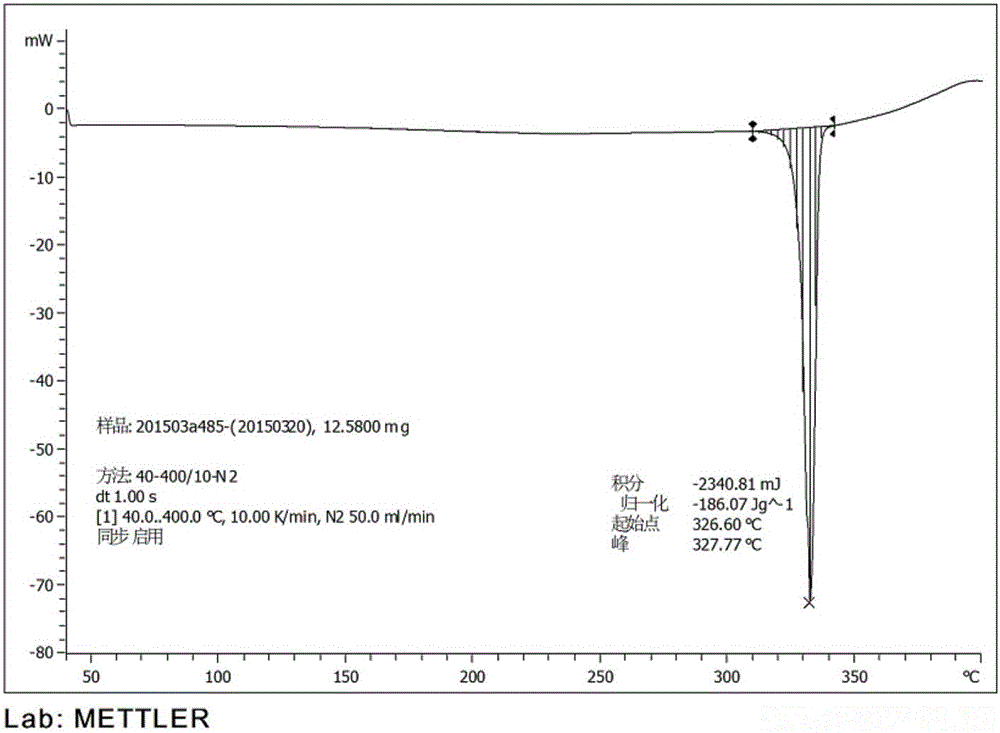
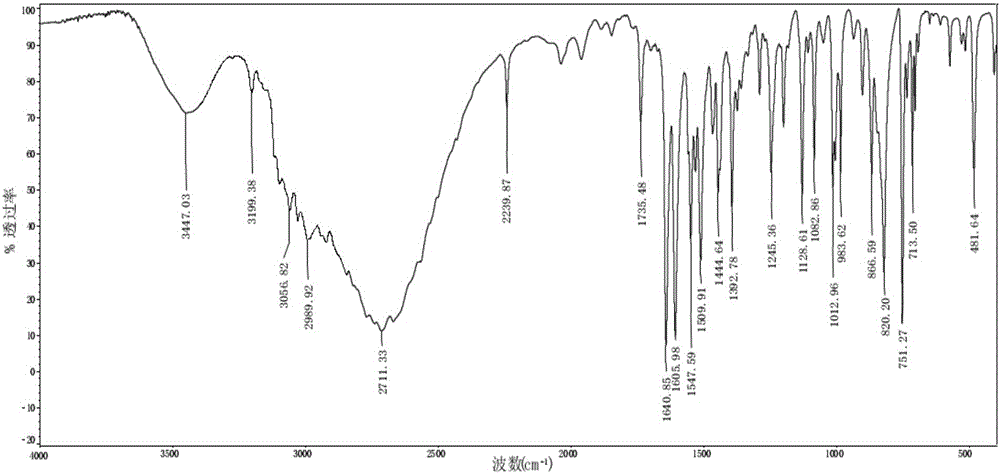
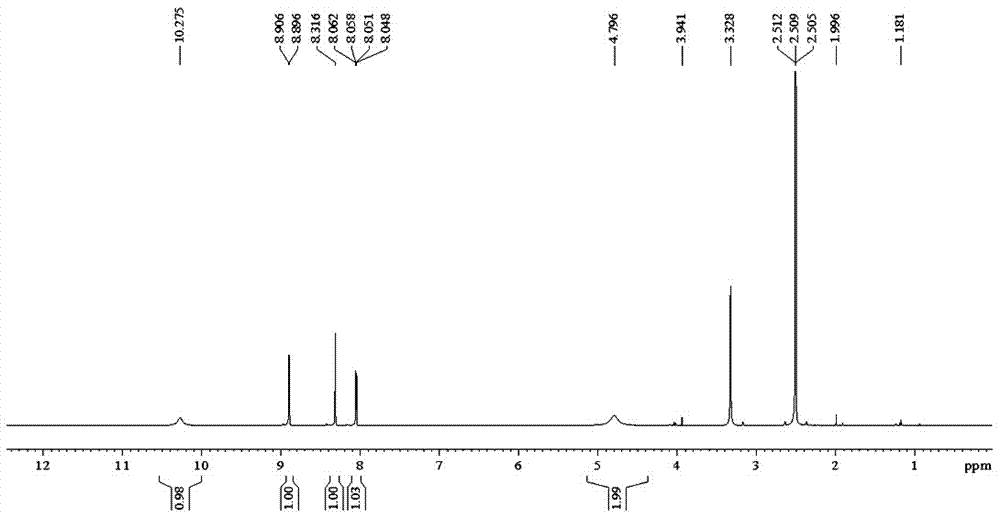
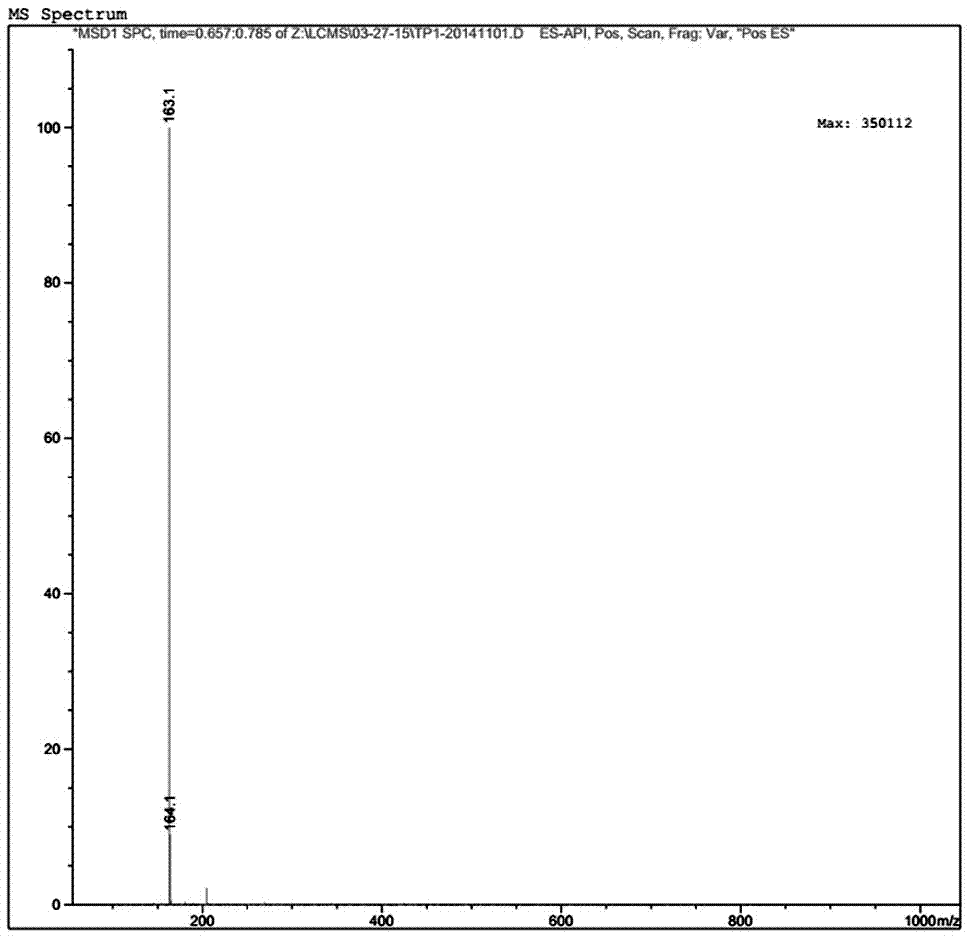
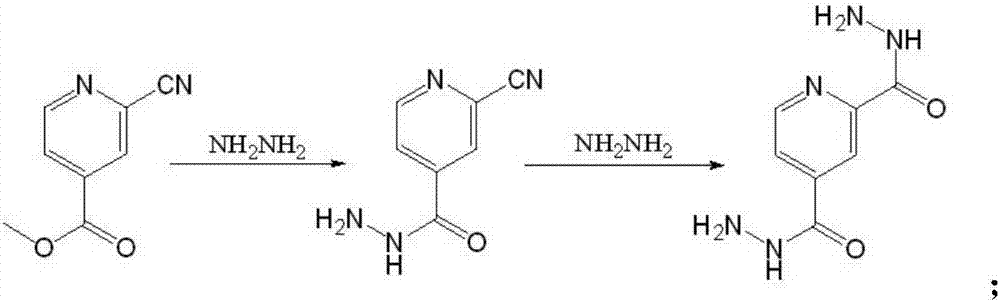
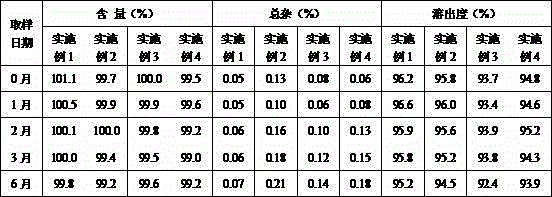
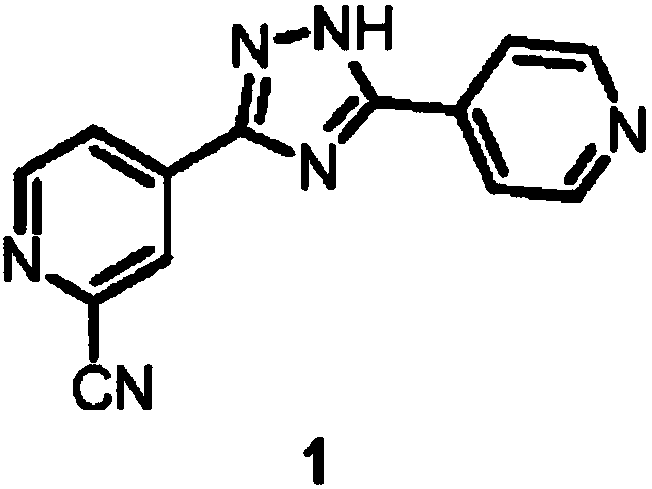
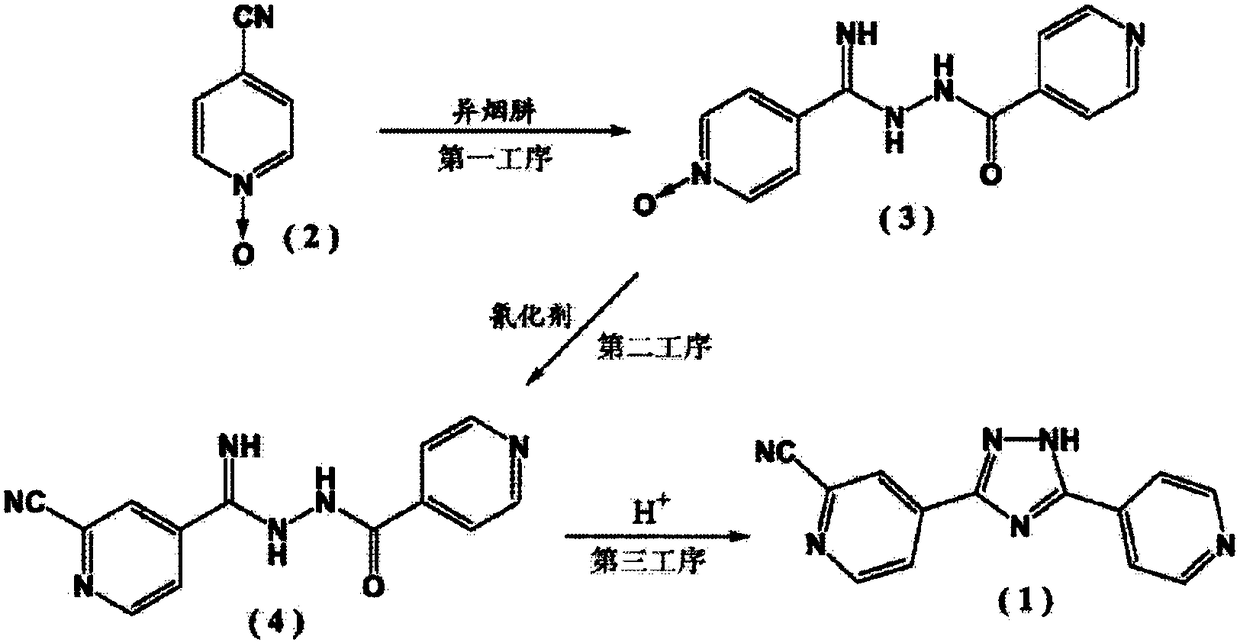
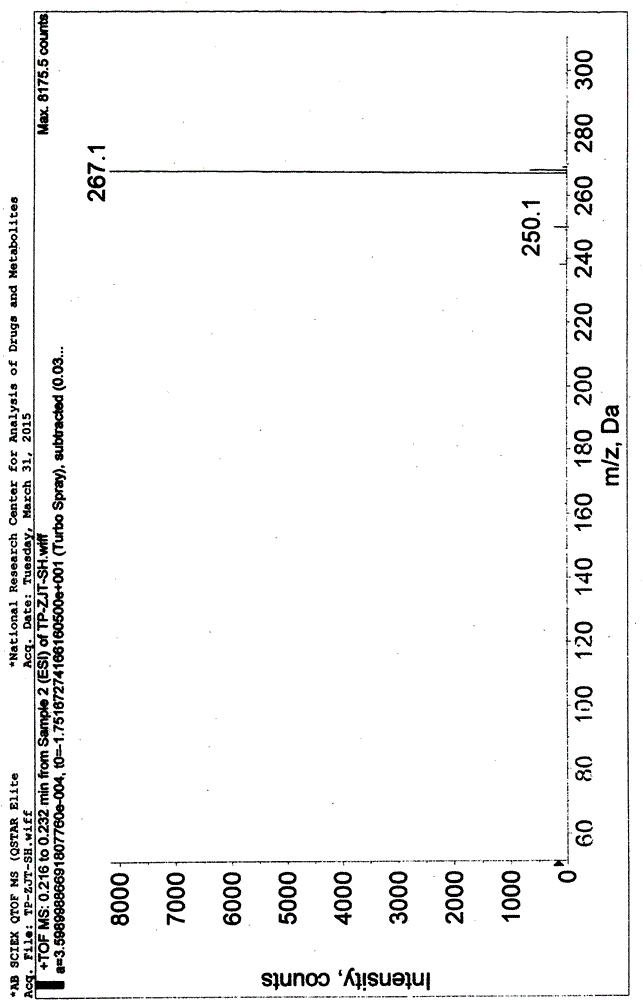
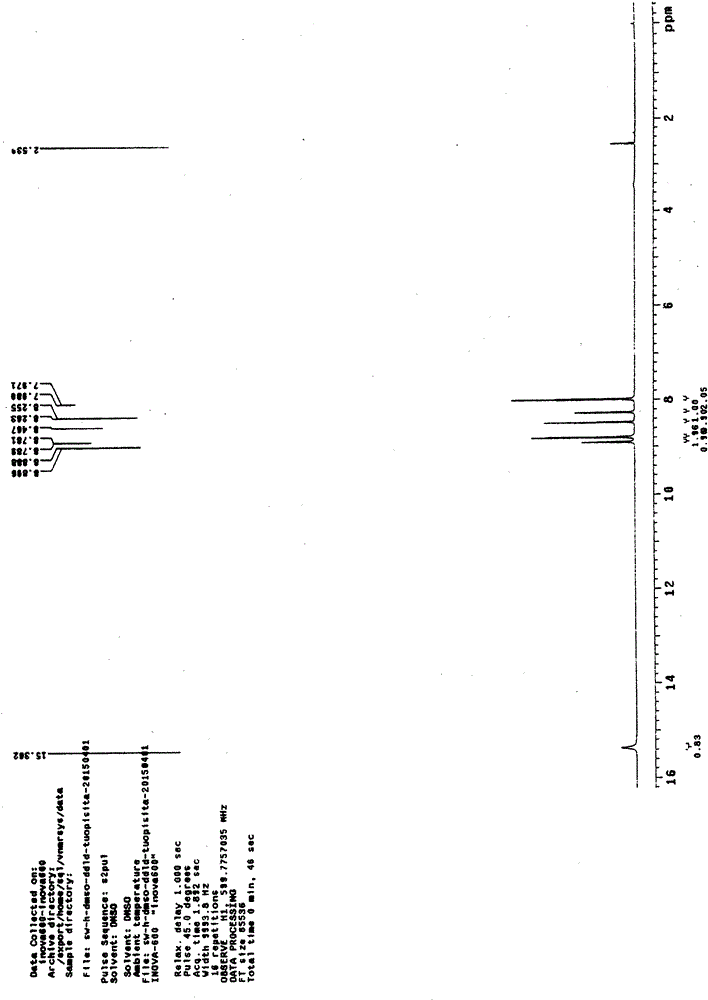
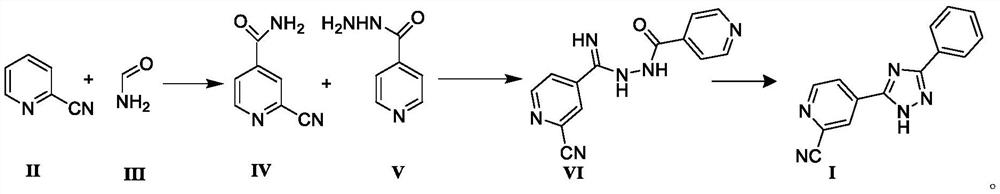
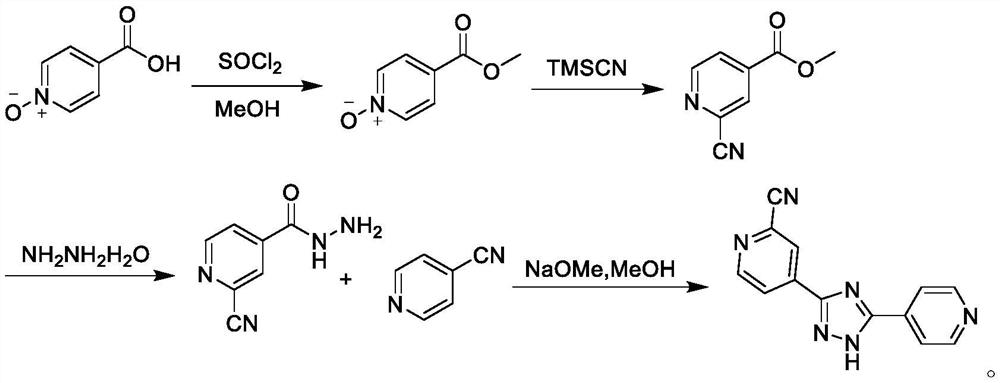
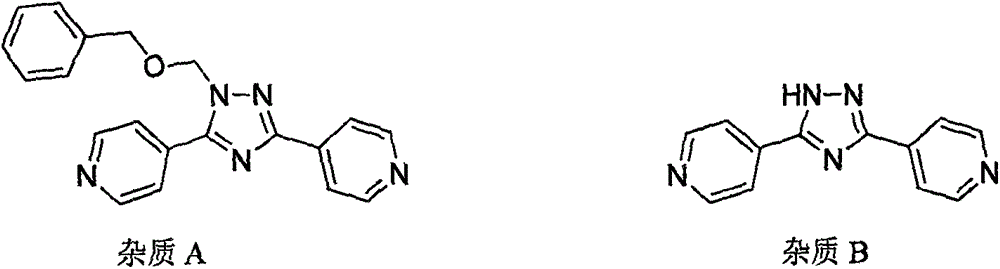
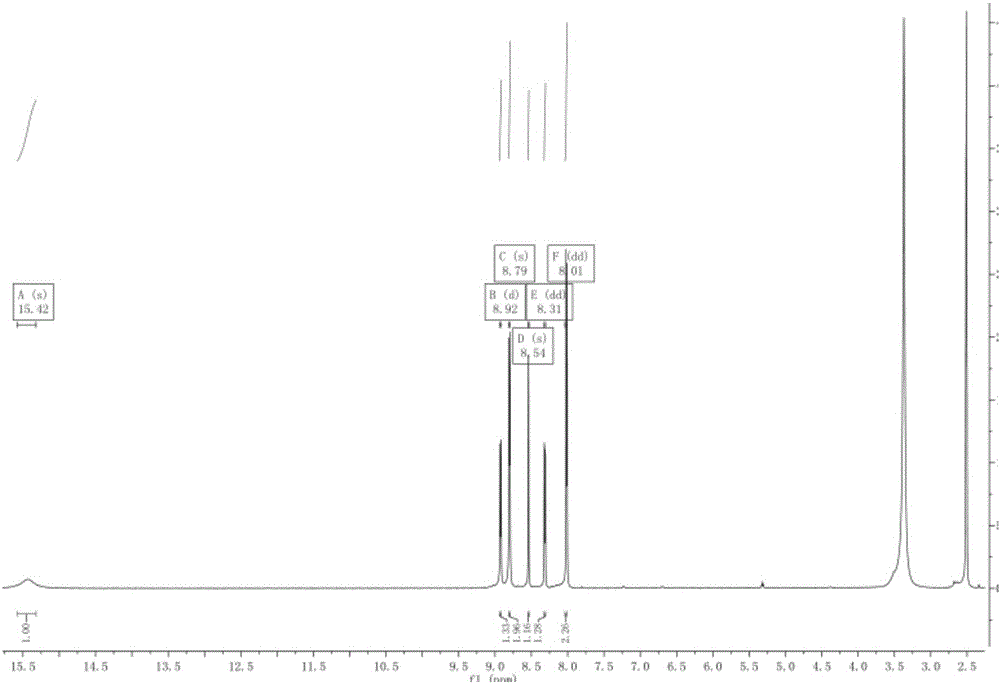
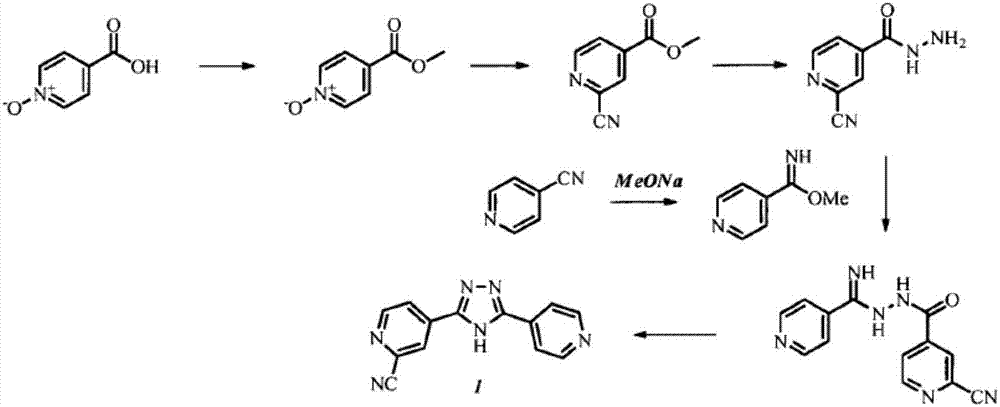
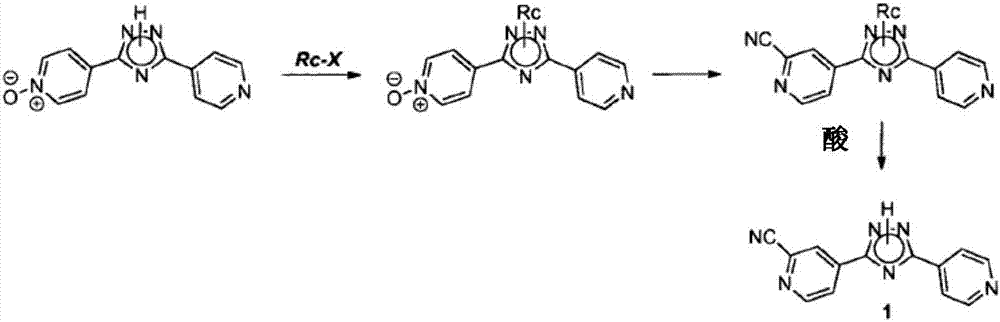
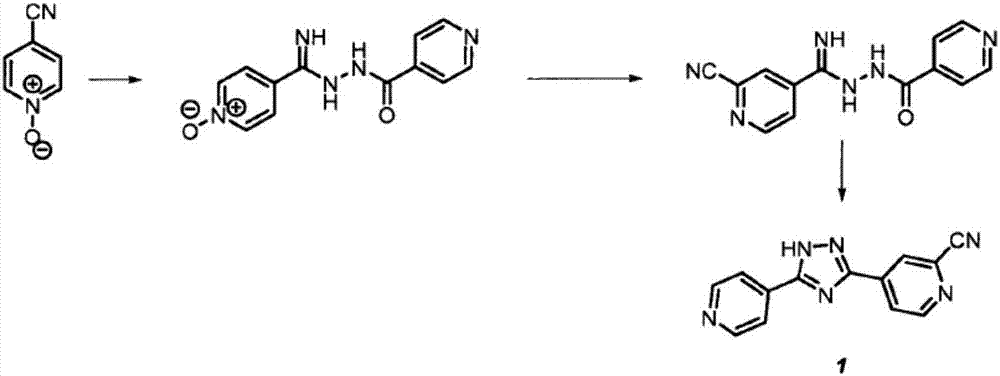
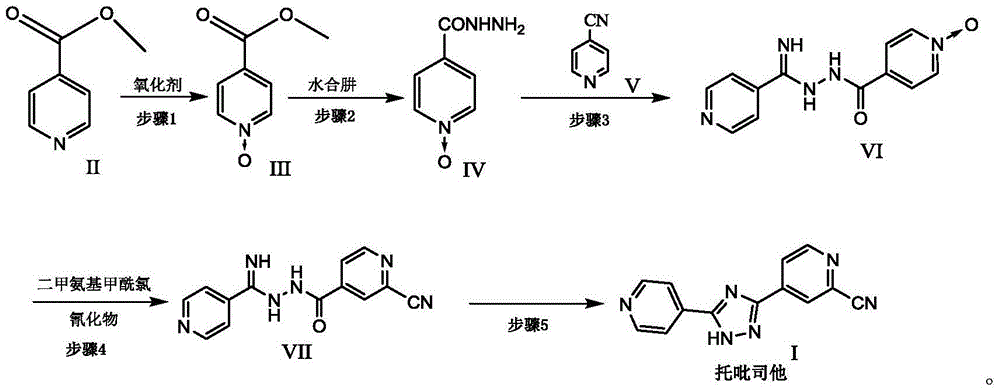
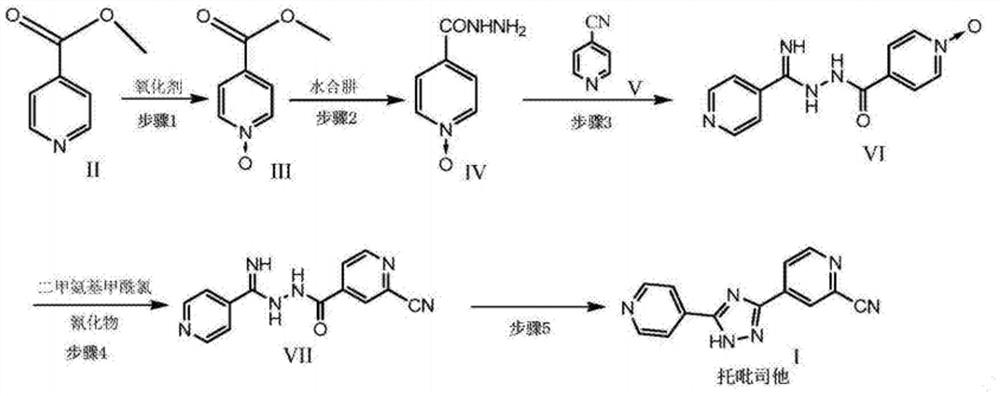
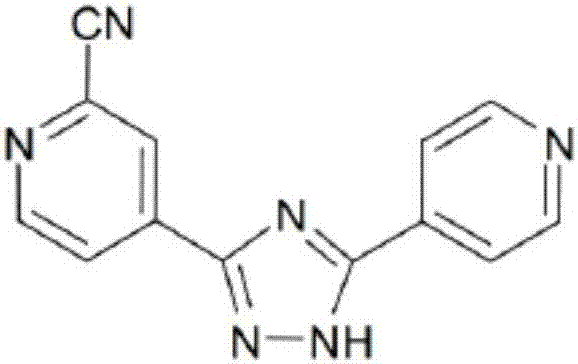
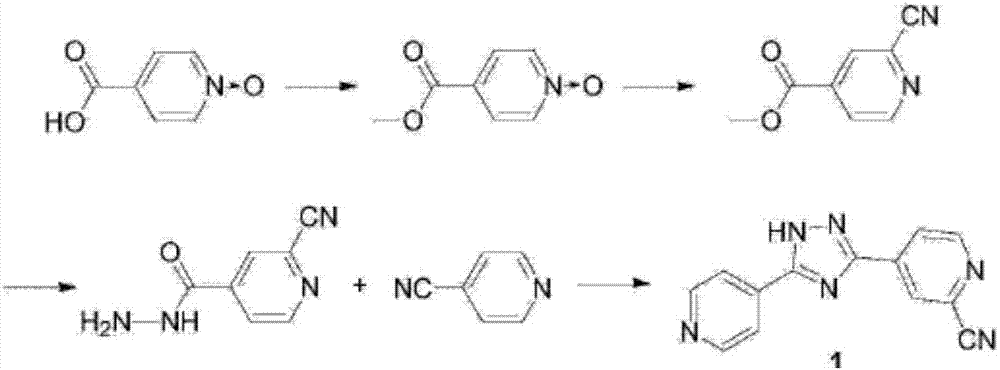
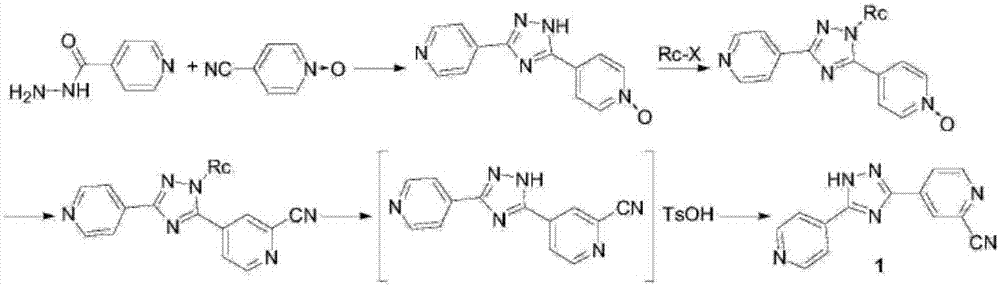
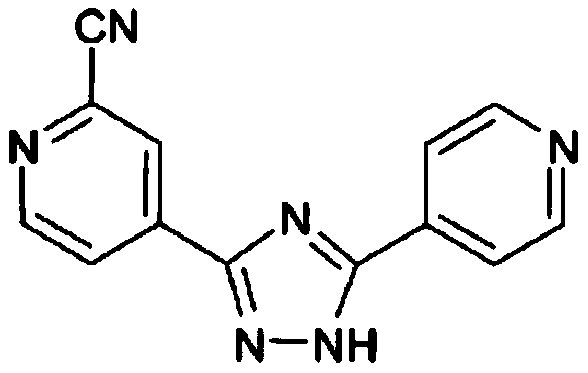
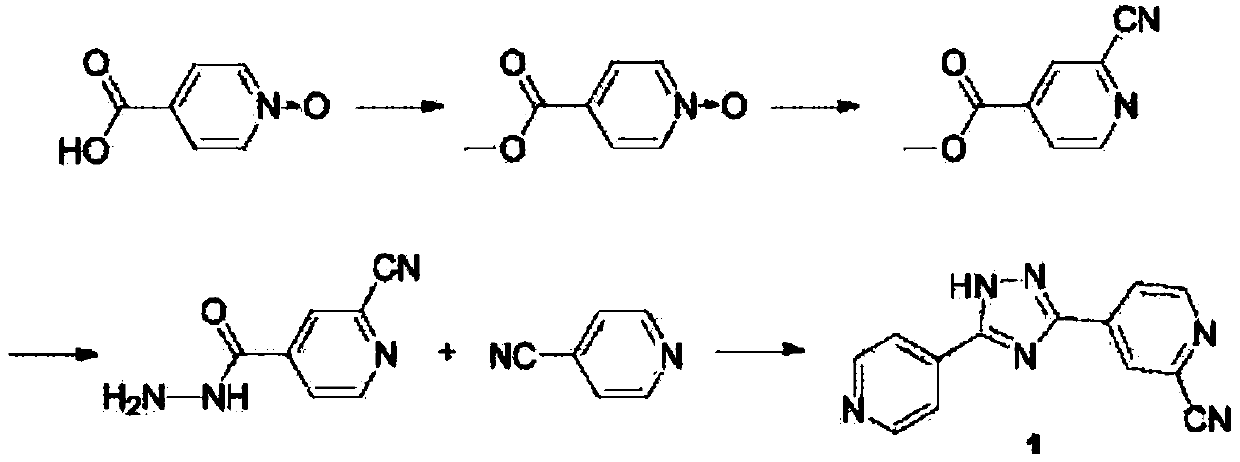
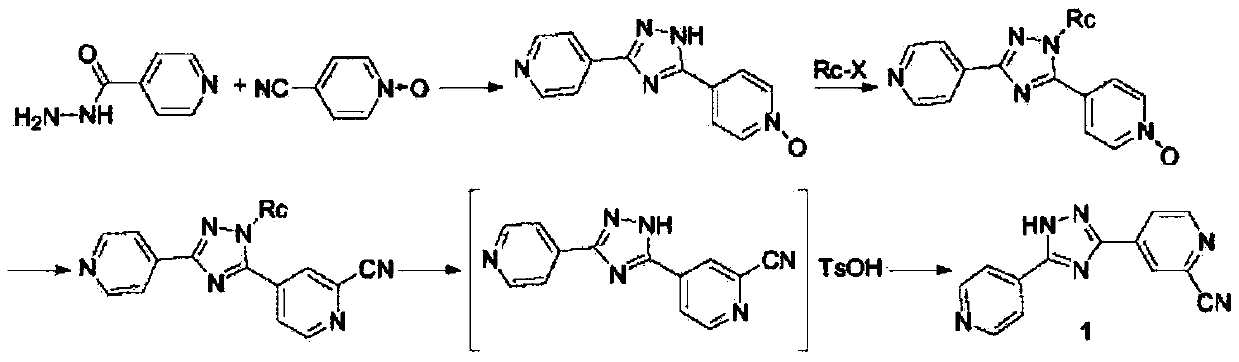
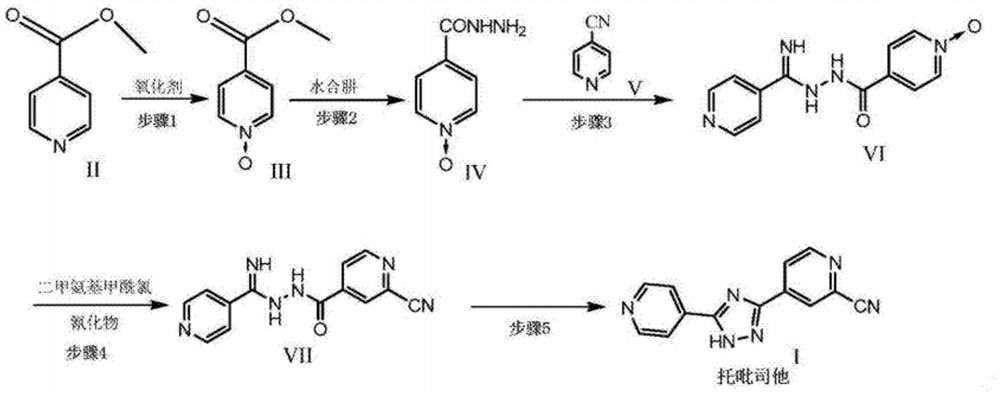
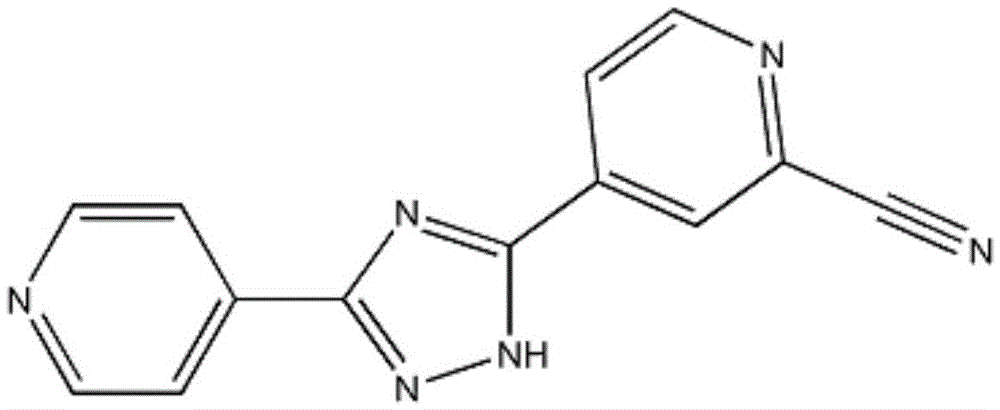
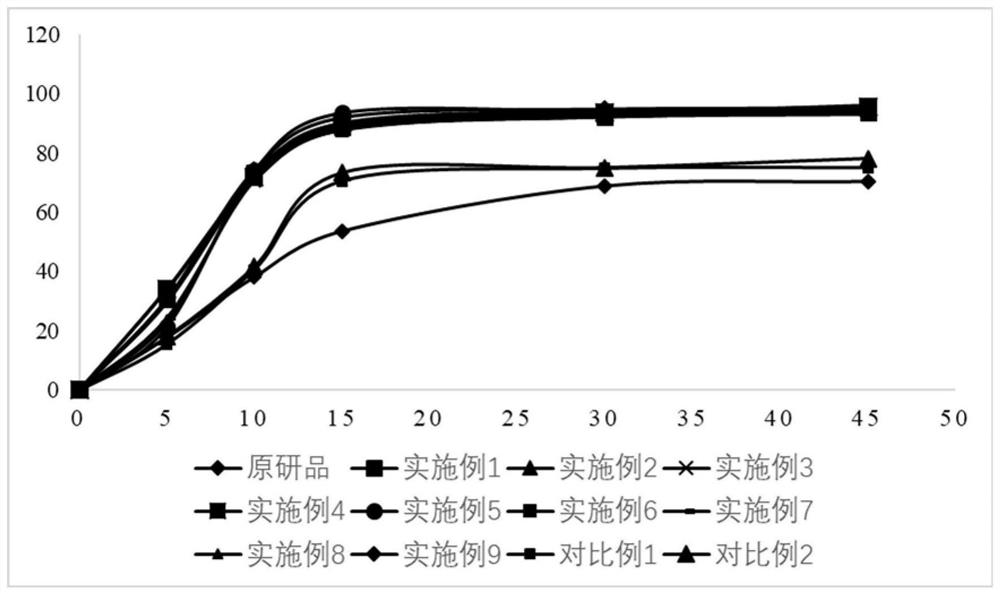
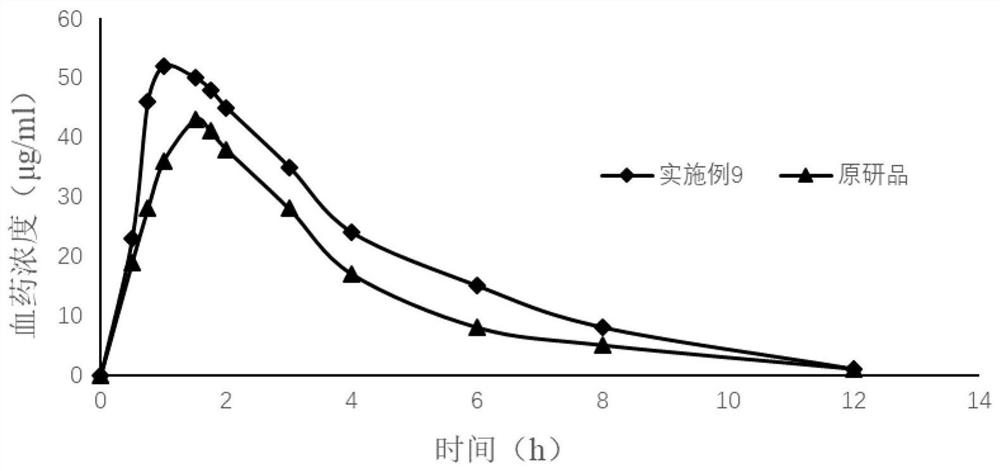
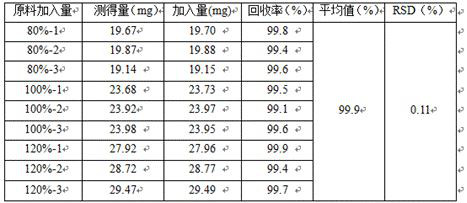
Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile
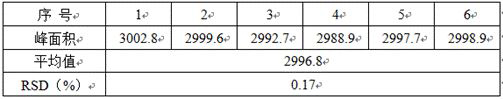
InactiveCN104151297AEasy to operateSimple processing capacityOrganic chemistryTopiroxostatDimethylcarbamyl chloride
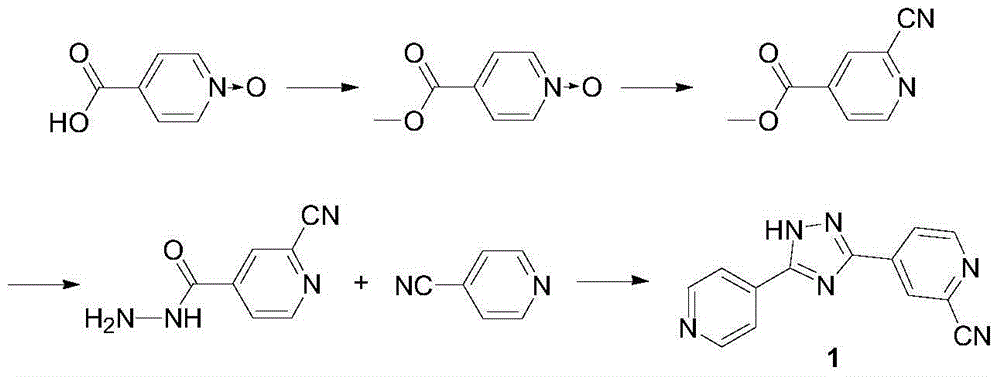
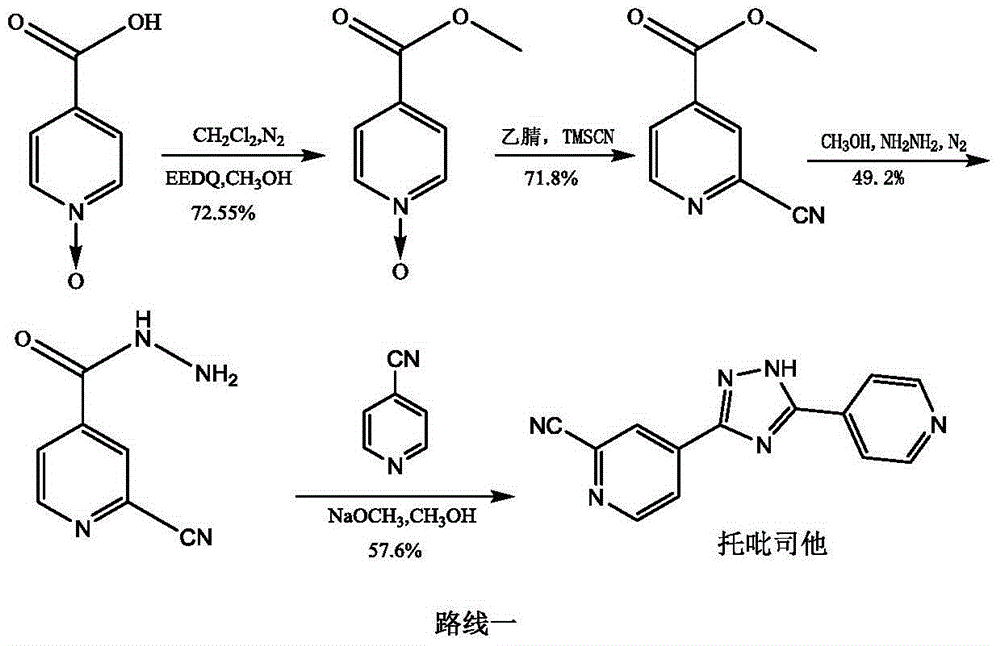
The invention relates to a preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile. The method includes following steps: preparing a compound methyl 2-cyanoisonicotinate represented as the formula (4) with a compound methylisonicotinic-N-oxide (5) being a starting raw material in the presence of a copper catalyst (CuX), a metal cyanide and dimethylcarbamyl chloride; performing hydrazinolysis to the methyl 2-cyanoisonicotinate to obtain a compound 2-cyanoisoniazide represented as the formula (3); and finally performing condensation with a compound 4-cyanopyridine represented as the formula (2) to obtain a compound topiroxostat represented as the formula (1). The method only comprises three steps and is simple in operation and post-treatment. By means of the copper catalyst, a usage amount of the metal cyanide is greatly reduced so that reaction conditions are milder. The method is high in purity of the prepared product and is suitable for industrial production.
Owner:王庆本 +1
Preparation method for topiroxostat
The invention belongs to the field of medicine and chemical engineering, and particularly relates to a preparation method for topiroxostat. 2-chloro-4-[(5-pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]-pyridine is subjected to a cyanation reaction under the action of a cyanation reagent in the presence of a catalyst, base and a ligand to obtain topiroxostat. The preparation method comprises the following steps: 4-cyanopyridine-N-oxide is taken as a starting material, 1,2-dichloroethane is taken as a solvent, triethylamine is taken as base, phosphorus oxychloride is used as a chlorinated reagent, and chlorination is conducted to obtain 2-chloro-4-cyanopyridine; 2-chloro-4-cyanopyridine and isoniazide are in a methanol solvent, sodium methoxide is taken as a catalyst, and close-loop condensation is performed to obtain 2-chloro-4-[(5-pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]-pyridine. The preparation method has the advantages that a safe and cheap cyanogroup source is selected, a hypertoxic cyanation reagent is avoided, the environmental harm is reduced, the product yield is high, the purity is high, and the suitability for industrial mass production is high.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Novel topiroxostat crystal form and method for preparing same
ActiveCN104961730AGood wet stabilityHigh humidity stabilityOrganic chemistrySolubilityActivated carbon
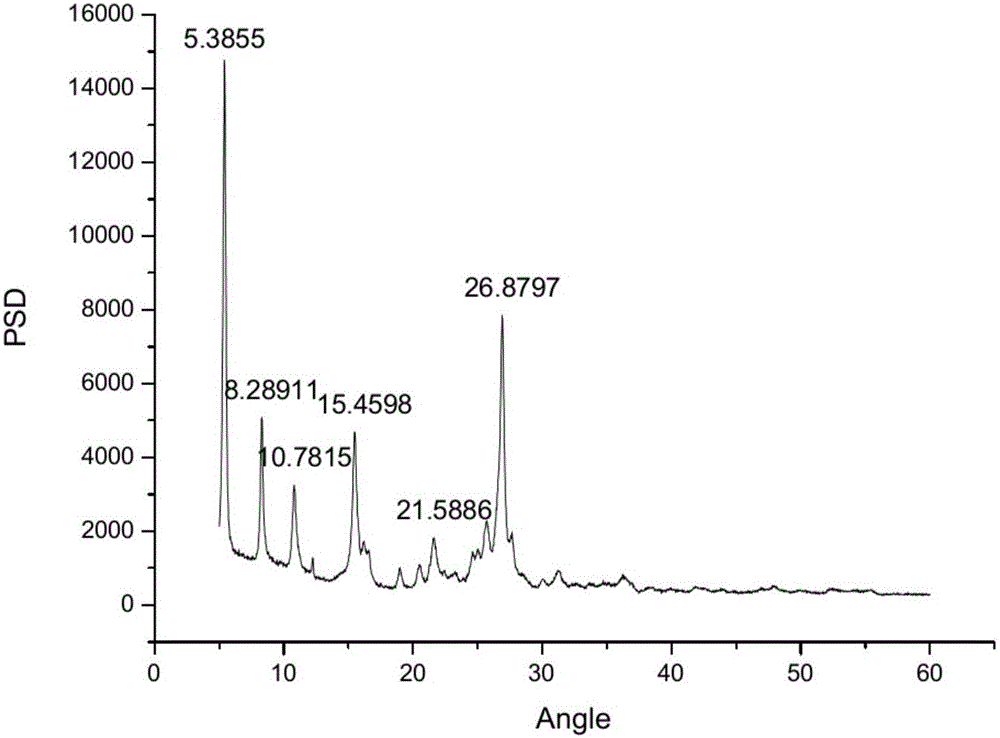
The invention belongs to the field of chemical pharmaceutical technologies, and particularly relates to a novel topiroxostat crystal form. 2-theta characteristic peaks in X-ray powder diffraction patterns of the novel topiroxostat crystal form are positioned at 5.38+ / -0.2, 8.29+ / -0.2, 10.78+ / -0.2, 15.46+ / -0.2, 21.59+ / -0.2 and 26.88+ / -0.2 degrees. The invention further provides a method for preparing the novel topiroxostat crystal form. The method includes adding topiroxostat into organic solvents; adding alkali into the organic solvents; stirring the topiroxostat and the alkali in the organic solvents until that solid clearly dissolves; adding activated carbon for color removal; filtering the organic solvents to obtain filter liquid; dripping acid in the filter liquid to adjust pH (potential of hydrogen) of the filter liquid until the pH of the filter liquid reaches 6-7; dissolving solid out of the filter liquid; performing extraction filtration on the filter liquid to obtain filter cakes; leaching the filter cakes; drying the filter cakes to obtain the novel topiroxostat crystal form. The novel topiroxostat crystal form and the method have the advantages that the novel topiroxostat crystal form is good in thermal stability and high in wet stability and solubility; products are high in purity, are quite suitable for long-term storage and are suitable for preparing solid preparations with good dissolution and / or stability; the products prepared by the aid of the method have the single and stable crystal form and are quite suitable for industrial production.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Preparation method of topiroxostat
ActiveCN107573330AReaction raw materials are readily availableThe reaction conditions are mild and easy to controlOrganic chemistryLoop closingTopiroxostat
The invention provides a preparation method of topiroxostat. The preparation method comprises the steps that 2-cyano methyl isonicotinate is used as raw materials; hydrazinolysis is performed at -10 DEG C to -20 DEG C to obtain an intermediate; the intermediate and 4-cyanopyridine react under the conditions with sodium ethoxide and the pH being 4 to 6 to obtain the topiroxostat. The preparation method has the advantages that the 2-cyano methyl isonicotinate is used as a starting material; the 2-cyano methyl isonicotinate and hydrazine hydrate take condensation reaction at low temperature to prepare the intermediate; the intermediate and the 4-cyanopyridine are subjected to condensation and loop closing under the acid condition with sodium ethoxide to prepare the topiroxostat. The raw materials can be easily obtained; the reaction conditions are mild and are easy to control; a reagent with high toxicity is not used in the reaction process; the released toxic substances are few; the sidereaction products are few; the reaction safety is high; the pollution is small; the obtained purity is high; the preparation method is suitable for industrial production.
Owner:HEBEI UNIV OF CHINESE MEDICINE +1
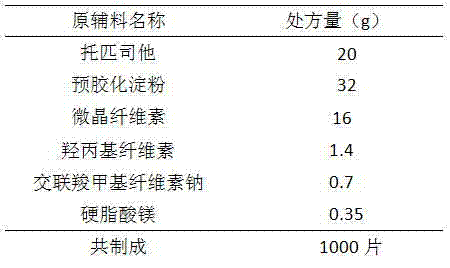
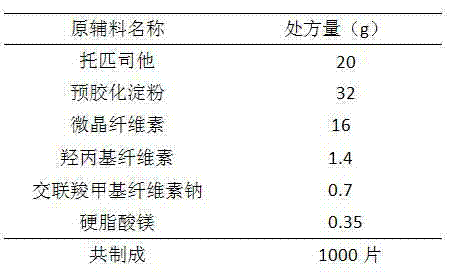
Topiroxostat tablet
The invention provides a topiroxostat tablet and a preparation method thereof, and belongs to the technical field of medicine. The invention relates to a topiroxostat clathrate compound dispersible tablet; the topiroxostat is firstly prepared into a clathrate compound and then the clathrate compound is prepared into the dispersible table. The topiroxostat tablet has the advantages of quick dissolving, quick absorption, high bioavailability, convenience for taking and the like.
Owner:CP PHARMA QINGDAO CO LTD
Topiroxostat oral preparation and preparation method thereof
InactiveCN104523690AThere are few varieties to chooseHigh dissolution rateOrganic active ingredientsSkeletal disorderTopiroxostatCombinatorial chemistry
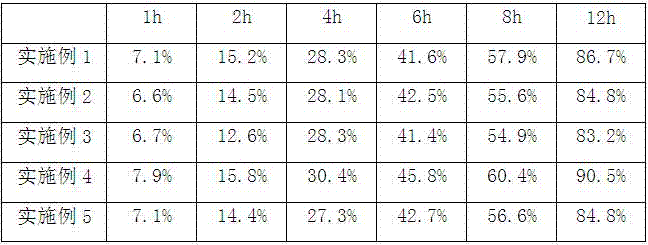
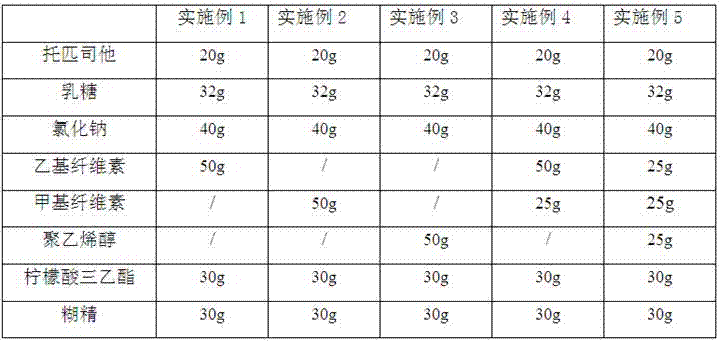
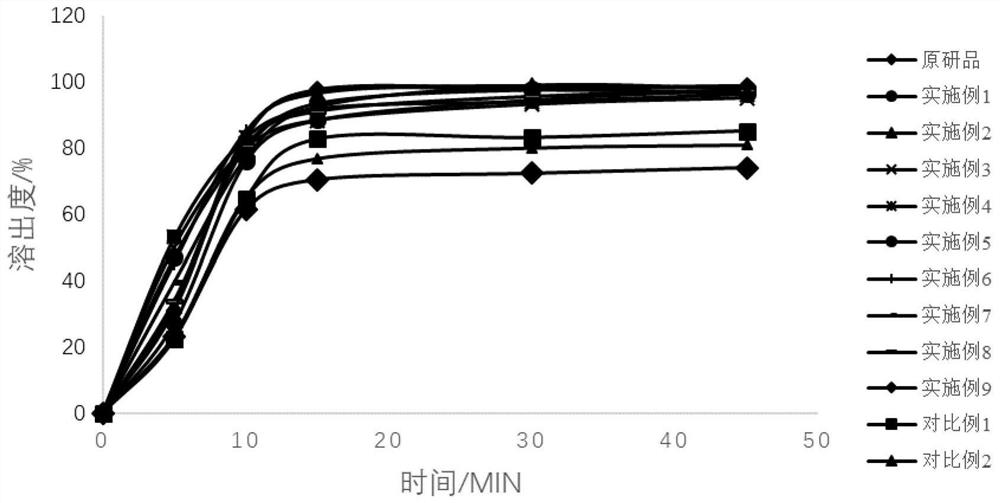
The invention provides a topiroxostat oral preparation and a preparation method thereof. The topiroxostat oral preparation is composed of an active ingredient topiroxostat as well as a filling agent, a disintegrating agent, a binding agent and a lubricating agent. The prepared topiroxostat oral preparation has no special requirement on particle size on the active ingredient, superfine grinding does not need to be carried out, energy consumption is low, dissolution rate reaches more than 95%, bioavailability is high, the defects of low dissolution rate and low bioavailability of the active ingredient are overcome, quality is stable and reliable, and a market development prospect is broad.
Owner:CHANGSHA BAISHUN BIOTECH
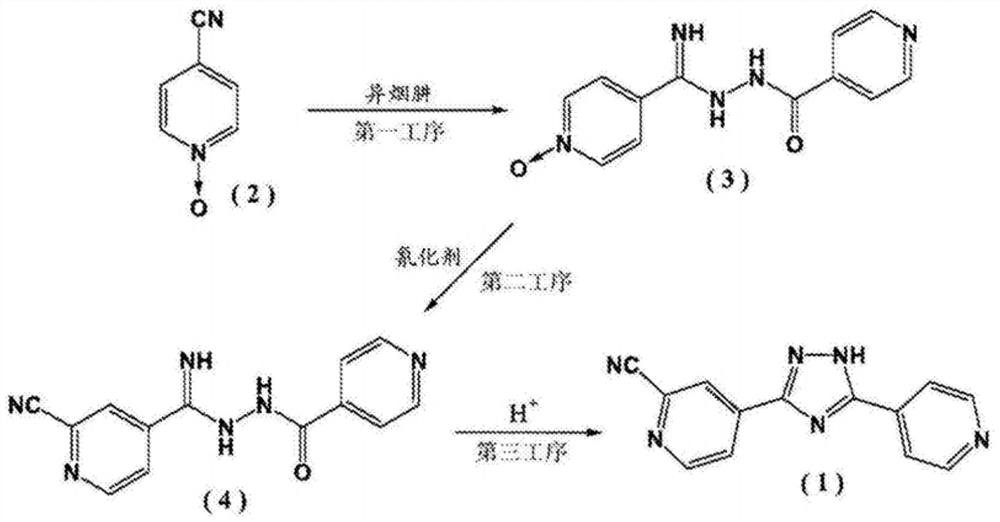
Topiroxostat and preparation method of intermediate of topiroxostat
InactiveCN108101840ARaw materials are cheap and easy to getThe reaction is easy to operateOrganic chemistryNitrogenTopiroxostat
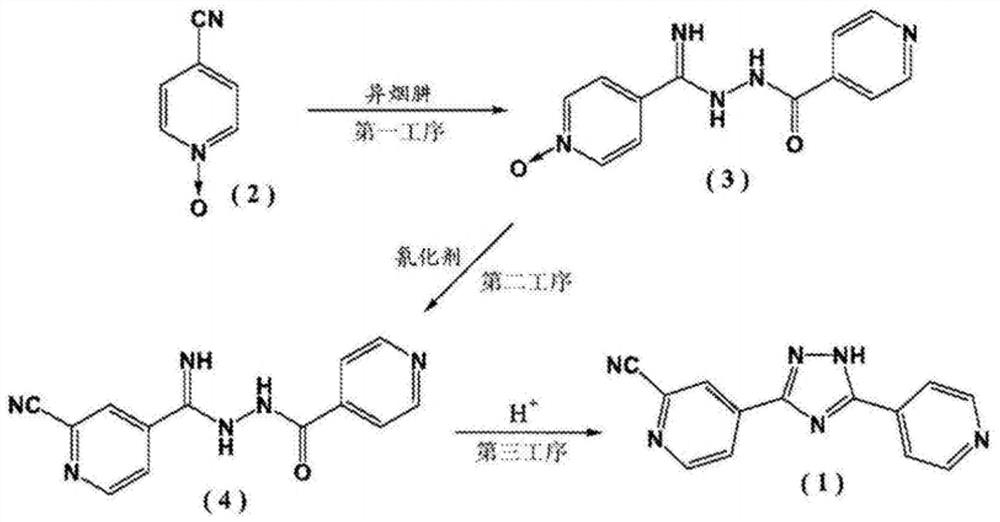
4-cyanopyridine is taken as a raw material, a compound III is obtained through a reaction, the compound III and a compound IIB undergo an amidation reaction to obtain a compound IV, and a ring-closurereaction is carried out in the function of an acid catalyst to obtain a target topiroxostat molecule. According to the scheme, the raw materials are cheap and are easy to obtain, the product qualityis high, the reaction is simple to operate and is mild, the yield is high, and the waste gas, waste water and industrial residue are less produced. In the reaction process, nitrogen protection isn't required, and a cyaniding reagent isn't used, so that the method has bright industrial prospects.
Owner:NANJING HUAWE MEDICINE TECH DEV
Topiroxostat tablet and preparation method thereof
ActiveCN104758263AReasonable choice of methodSimple process routeOrganic active ingredientsNervous disorderXanthine oxidase inhibitorXanthine
The invention provides a topiroxostat tablet and a preparation method thereof, and belongs to the technical field of medicines. The topiroxostat tablet is mainly prepared from a main drug, filler, a disintegrating agent, an adhesive and a lubricant. Topiroxostat is a non-purine selective xanthine oxidase inhibitor, and is used for treating gout and hyperuricemia. The topiroxostat tablet is safe and effective, has the advantages of quick disintegration, good solubility, stable quality, high bioavailability and low cost, is convenient to take, and can be used for improving the compliance of patients.
Owner:CP PHARMA QINGDAO CO LTD
Preparation process and method for topiroxostat
InactiveCN105294656AGuaranteed homeostasis responseQuality improvementOrganic chemistryTopiroxostatReagent
The invention relates to a preparation process and method for topiroxostat. 4-cyanopyridine [formula (II)] and 2-cyanoisonicotinohydrazide [formula (III)] are subjected to a condensation reaction by stages to prepare [formula (IV), 4-pyridylcarbonylhydrazine-Nminute-(2-cyanopyridine-4- carbimide], washing the [formula (IV)] and then carrying out a cyclization reaction to prepare the topiroxostat [(formula (I)]. The preparation process disclosed by the invention is simple and convenient, free of special toxic reagent, green and environment-friendly, good in product yield, good in quality and in conformity with the medicinal requirement.
Owner:大道隆达(北京)医药科技发展有限公司
Preparation method of topiroxostat
PendingCN113666909ASimple and efficient operationHigh reaction yieldOrganic chemistryPtru catalystAcyl group
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a preparation method of topiroxostat. The preparation method disclosed by the invention comprises the following steps: 2-cyanopyridine and formamide are taken as raw materials to react to prepare 2-cyano-4-carbamoyl-pyridine; the 2-cyano-4-carbamoyl-pyridine continues to react with isoniazide to obtain a key intermediate 4-picolinic acid hydrazide-N'-(2-cyanopyridine-4-carbodiimide), and the key intermediate 4-picolinic acid hydrazide-N'-(2-cyanopyridine-4-carbodiimide) is subjected to ring closing to obtain the topiroxostat. The invention provides the novel method for synthesizing topiroxostat, which avoids the use of highly toxic chemical reagents, replaces a traditional catalyst with a green catalyst, is milder in reaction, is economical and environment-friendly, is higher in yield, and is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
High-dissolution rate topiroxostat pharmaceutical composition and preparation method thereof
InactiveCN104523691AThere are few varieties to chooseHigh dissolution rateOrganic active ingredientsSkeletal disorderPharmaceutical drugTopiroxostat
The invention provides a high-dissolution rate topiroxostat pharmaceutical composition and a preparation method thereof. The topiroxostat pharmaceutical composition comprises an active ingredient topiroxostat, and pharmaceutical adjuvants including a water-soluble solid dispersing carrier, a disintegrating agent and a lubricant. The prepared topiroxostat pharmaceutical composition does not have special requirements for the particle size of the active ingredient and does not need superfine grinding, so that the energy consumption is low, the dissolution rate is above 95%, the bioavailability is high, the defect that the active ingredient is bad in solubility and low in bioavailability is solved, the quality is stable and reliable and the market development prospect is good.
Owner:CHANGSHA BAISHUN BIOTECH
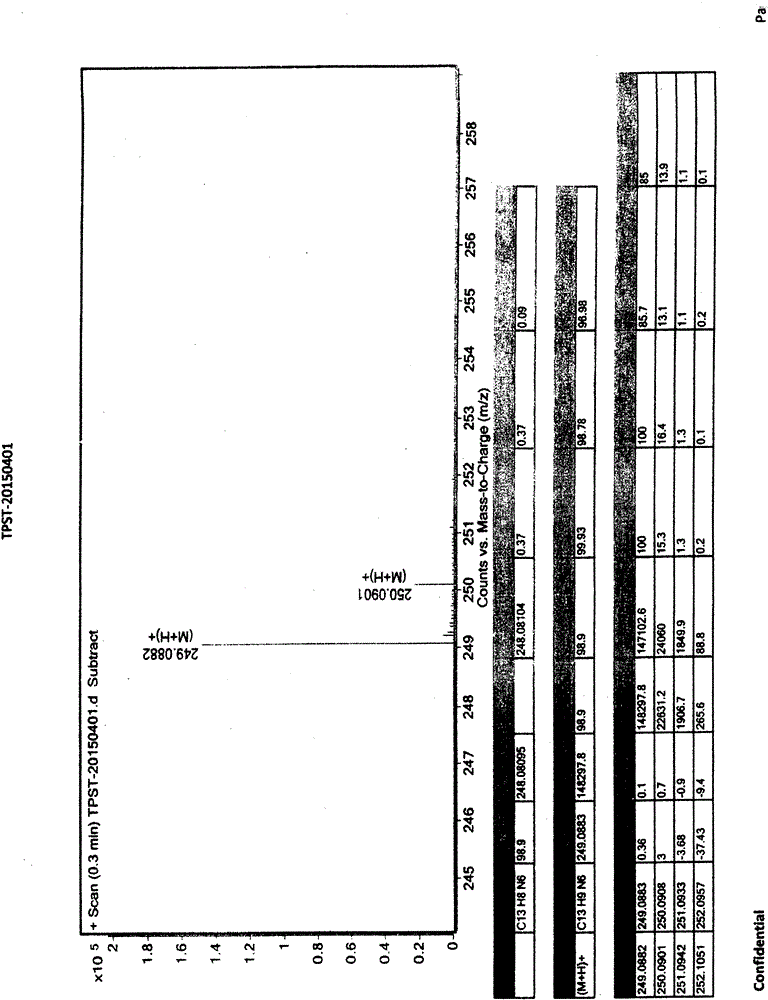
Preparation method of high-purity topiroxostat
The invention relates to the field of organic chemistry and pharmaceutical chemistry, specifically to a preparation method of topiroxostat. The technical scheme of the preparation method comprises a step I, dissolving a crude product (an oily product) of a compound I in an alcohol solvent or a mixed solution of an alcohol solvent and another solvent, raising the temperature to a reflux temperature, performing stirring and dissolving, slowly dropwise adding water till a few solid is precipitated, performing thermal insulation and stirring, reducing the temperature to 0 DEC C, performing stirring, carrying out cooling for crystallization, and performing filtration and drying to obtain a compound I with high purity; a step 2, adding the compound I obtained in the step I into a mixed solution of toluene and isopropanol, raising the temperature to 80 DEG C for solution, dropwise adding concentrated hydrochloric acid, performing thermal insulation and stirring, performing cooling to reach the room temperature, and performing drying at 60 DEG C to obtain topiroxostat hydrochloride; and a step 3, preparing topiroxostat. According to the preparation method of high-purity topiroxostat, concentrated hydrochloric acid instead of p-toluenesulfonic acid is used, and through purification of the intermediate compound I, the high-purity compound I is obtained, so that high-purity topiroxostat is prepared.
Owner:北京满格医药科技有限公司
One-pot method for synthesizing Topiroxostat
The invention discloses a one-pot method for synthesizing Topiroxostat. The one-pot method comprises the following steps: dissolving 2-cyano methyl isonicotinate into a solvent, reacting with hydrazine hydrate to generate an intermediate 2-cyanoisoniazide, then adding alkali for reaction in the same reactor, then adding 4-cyanopyridine to form a ring, and finally purifying to obtain the Topiroxostat. The one-pot method is short in technological process, simple in operation, high in raw material utilization rate and low in production cost, and has higher production and practical value.
Owner:NANJING UNIV OF TECH
Methods for the preparation of topiroxostat and intermediates thereof
The present invention relates to novel preparation methods for Topiroxostat through novel intermediates comprising novel methods for the formation of the triazole ring and for cyanation of the pyridylring.
Owner:PHARMATHEN
Preparation method of topiroxostat
The invention provides a preparation method of topiroxostat. The method comprises the following steps: subjecting isonicotinic acid as a starting material to oxidation with hydrogen peroxide to obtainisonicotinic acid-nitric oxide (an intermediate 1); then esterifying with methanol to obtain methyl isonicotinate-nitric oxide (intermediate 2); then performing hydrazinolysis with hydrazine hydrateto obtain isoniazide-nitric oxide (intermediate 3); then reacting with 4-cyanopyridine to obtain an intermediate 4; then cyaniding with trimethylsilyl cyanide to obtain an intermediate 5; and finallyperforming dehydration and cyclization to generate topiroxostat. According to the method, initial raw materials and reagents are cheap and easily available; experimental operation is simple and controllable, extreme reaction conditions are avoided, and the method is suitable for laboratory development and even industrial production; the total yield is high, and the production cost is reduced; purity of the finished product can be ensured.
Owner:孙哲
Topiroxostat tablet and preparation method thereof
InactiveCN105456209AGranulation went wellIncrease productivityOrganic active ingredientsSkeletal disorderDrug contentCurative effect
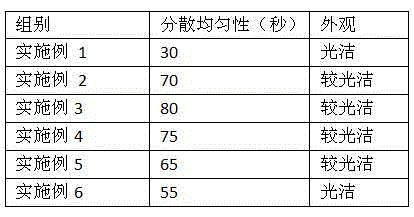
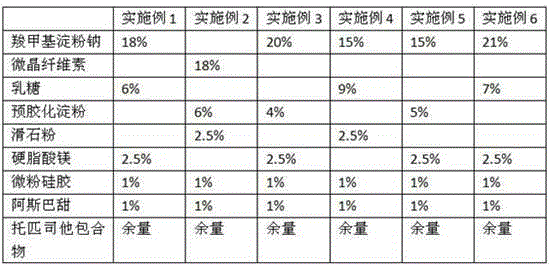
The invention relates to a topiroxostat tablet. The topiroxostat tablet is prepared from, by weight, 5%-60% of raw material topiroxostat, 10%-80% of auxiliary material filler, 1%-30% of disintegrating agent, 0.1%-5% of bonding agent and 0.2%-5% of lubricating agent. The topiroxostat tablet has the advantages that the topiroxostat tablet is prepared by adding an appropriate amount of the auxiliary material to the topiroxostat subjected to air jet pulverization, the drug content uniformity is good, dissolution is rapid and safe, the quality is stable, the topiroxostat tablet is suitable for industrial production, and the bioavailability is improved, so that the curative effect is improved, and the topiroxostat tablet is convenient to store and use.
Owner:SHANGHAI STEPPHARM CO LTD
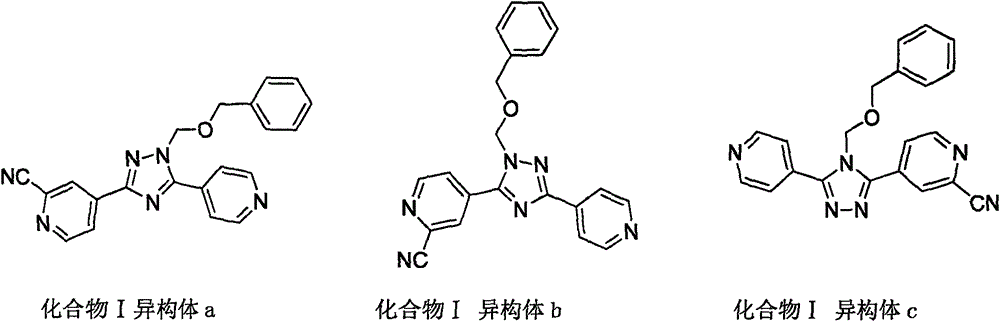
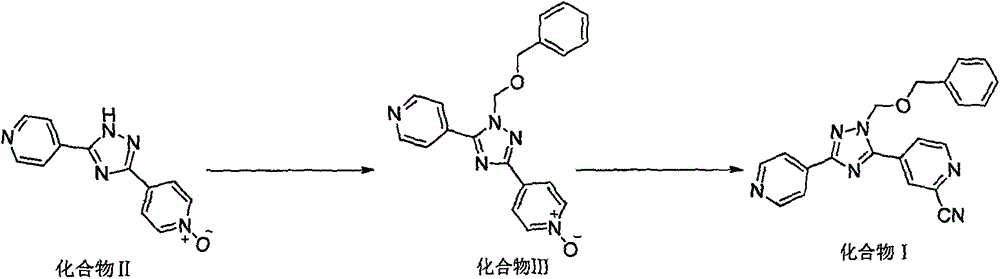
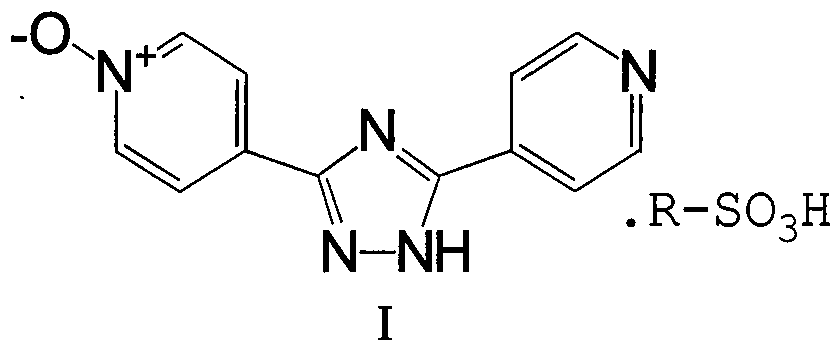
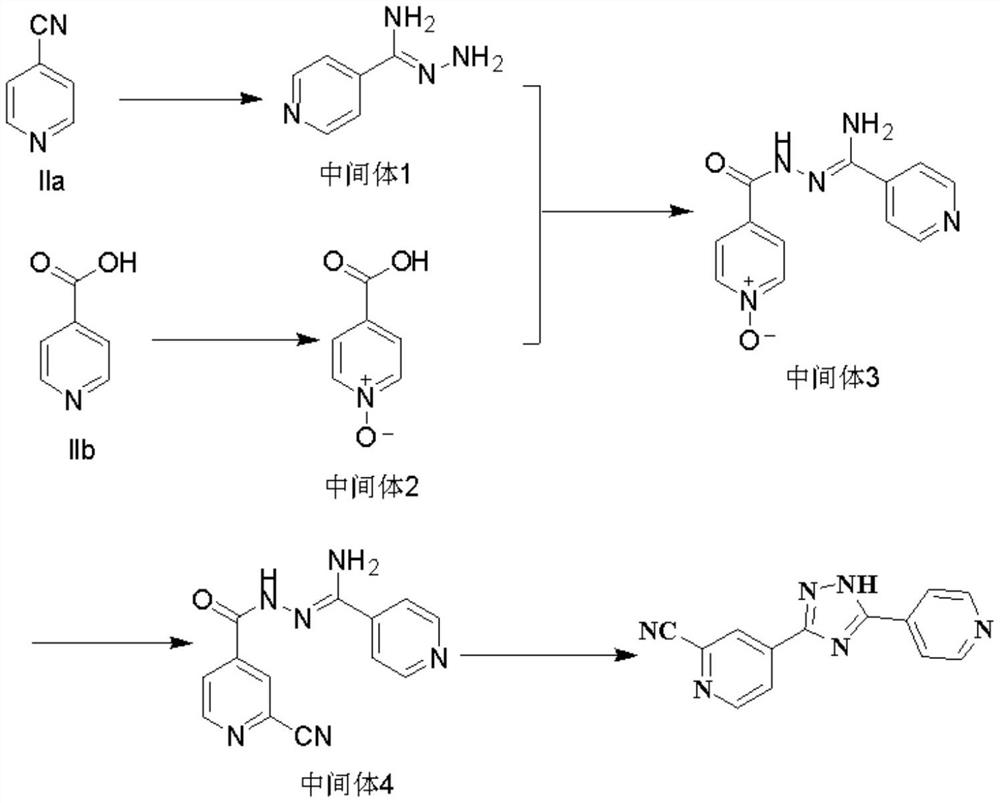
Intermediate for topipitastat, preparation method thereof, and method for preparing topipitastat from intermediate
The invention belongs to the technical field of medicines, and particularly relates to an intermediate for topipitastat, a preparation method thereof, and a method for preparing topipitastat from theintermediate. The intermediate for the topipitastat is 3-(4-pyridyl)-5-(1-oxo-4-pyridyl)-1,2,4-triazole p-toluenesulfonate, and the structural formula of the intermediate is represented by formula (I). The preparation methods are characterized in that methyl isonicotinate oxynitride and hydrazine hydrate undergo a condensation reaction, a ring closing reaction and a salt formation reaction to obtain the intermediate, and the intermediate and N,N-dimethylformyl chloride undergo a cyanation reaction and a refining reaction to obtain the topipitastat. The methods effectively solve the technical problems of complex preparation process and low purity of topiroxostat, and have the advantages of simple process, good reproducibility, low cost, environmental protection and energy saving, so the methods have high industrial values and remarkable social and economic benefits. The preparation methods are used for preparing the key intermediate for the topiroxostat and the topiroxostat.
Owner:BEIJING CHENG JI PHARMA
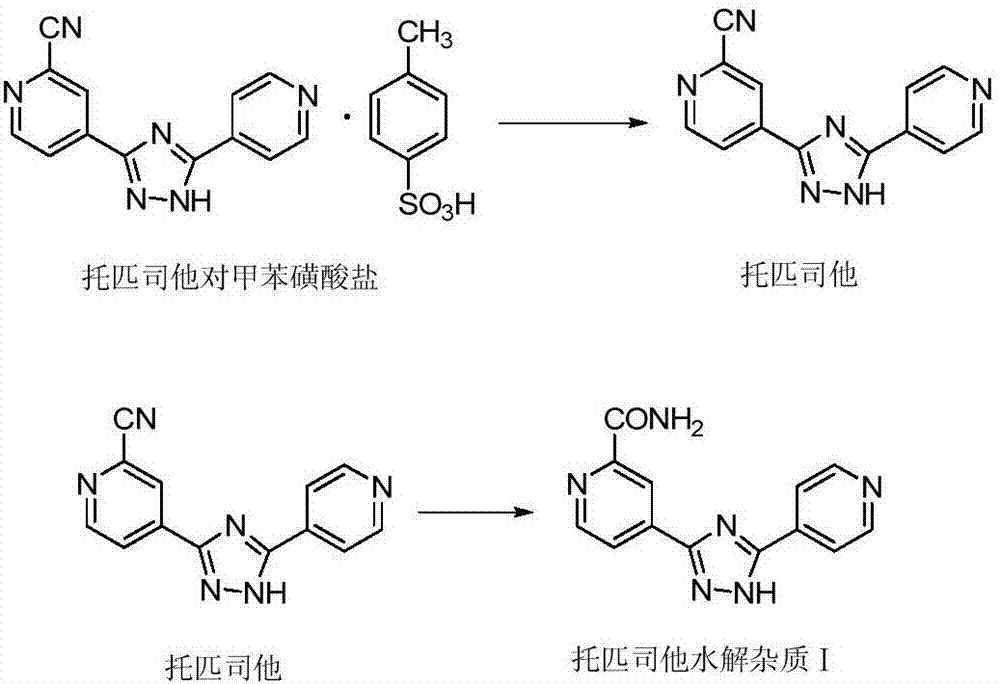
Method for preparing high-purity topiroxostat
The invention relates to a method for preparing high-purity 4-[5-(pyridin-4-yl)-1H-1,2,4-triazol-3-yl] pyridine-2-carbonitrile (namely topiroxostat). According to the method, the content of hydrolytic impurities of the topiroxostat can be effectively controlled, so that the prepared topiroxostat is controllable in quality and high in stability. The method mainly comprises the following technical steps: 1) dissolving 4-[5-(pyridin-4-yl)-1H-1,2,4-triazol-3-yl] pyridine-2-carbonitrilep-toluenesulfonate by using a mixed solvent, adding alkali diluted by the mixed solvent at a room temperature, and enabling the solution to be completely clarified, wherein the mixed solvent is formed by alcohol and water; 2) rapidly adding the dosed inorganic acid into the obtained solution, and gradually separating out white crystals; and 3) filtering, washing, and drying, thereby obtaining the product.
Owner:SHANDONG XINHUA PHARMA CO LTD
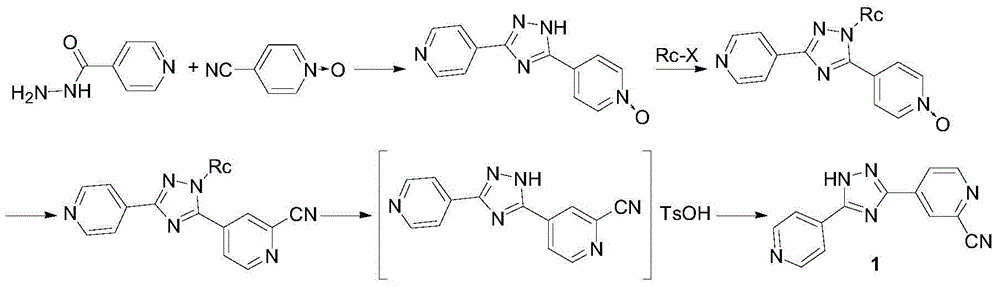
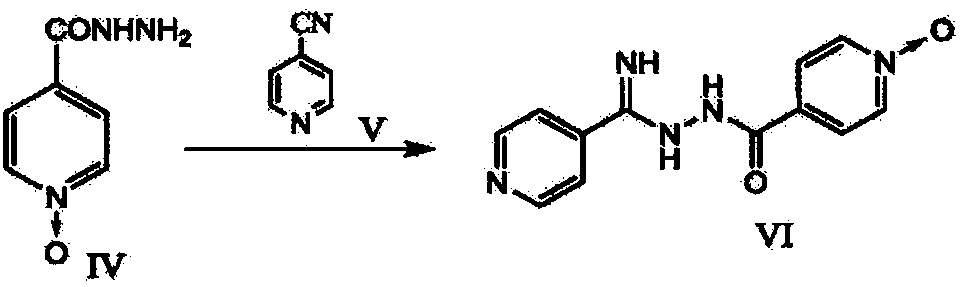
New topiroxostat synthesis intermediate and preparation method thereof
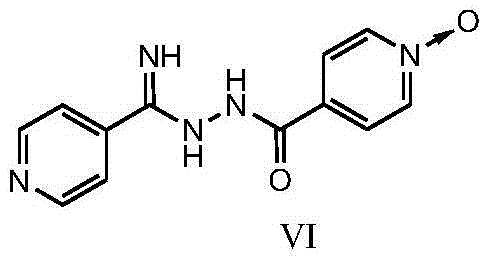
The present invention provides a new topiroxostat synthesis intermediate 4-(2-(imino(pyridine-4-yl)methyl)hydrazinocarbonyl)pyridine N-oxide (compound VI) and a preparation method thereof, wherein isoniazid N-oxide IV and 4-cyanopyridine V are subjected to a reaction in a suitable solvent under an alcohol alkali condition to obtain the product, the alcohol alkali is selected from sodium methoxide, sodium ethoxide, potassium ethoxide or potassium t-butoxide, and the reaction formula is defined in the specification. According to the present invention, through the compound VI, the gout treating drug topiroxostat can be prepared under the mild and easy industrial control reaction conditions.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Synthesis method of topiroxostat
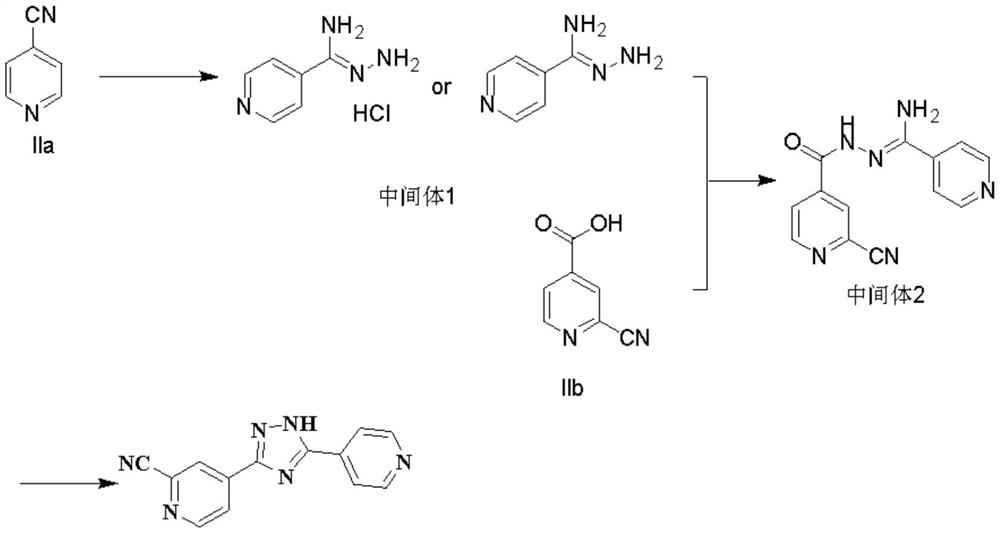
The invention provides a synthesis method of topiroxostat, and relates to the technical field of medicine synthesis. The synthesis method of topiroxostat comprises the steps of carrying out heat-preservation stirring reaction on a raw material 4-cyanopyridine and 80% hydrazine hydrate in the presence of an alcohol solvent and an alkaline reagent, and carrying out post-treatment to obtain an intermediate 1, or carrying out post-treatment under the condition of hydrochloric acid to obtain a hydrochloride form of the intermediate 1; reacting the intermediate 1 or the hydrochloride form of the intermediate 1 with 2-cyano-4-picolinic acid in the presence of a solvent and a condensing agent, and performing post-treatment to obtain an intermediate 2; and heating and refluxing the intermediate 2 for 2-4 hours under the condition of acetic acid, cooling to room temperature, filtering, and carrying out forced air drying to obtain the topiroxostat (crystal form I). The synthesis method has the advantages of low production cost, high yield, high purity and less three wastes, and is suitable for industrial production of topiroxostat and intermediates thereof.
Owner:南京安一合医药科技有限公司
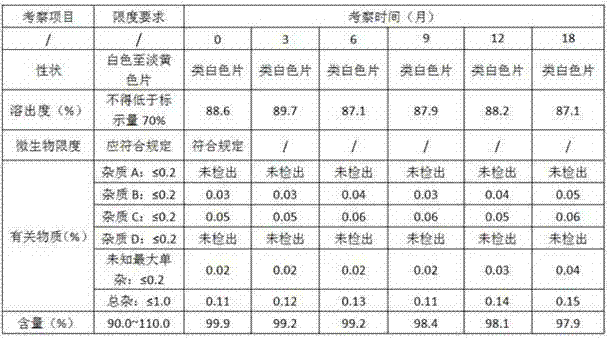
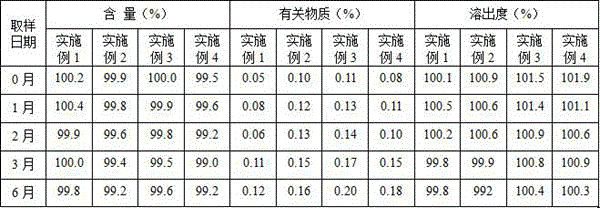
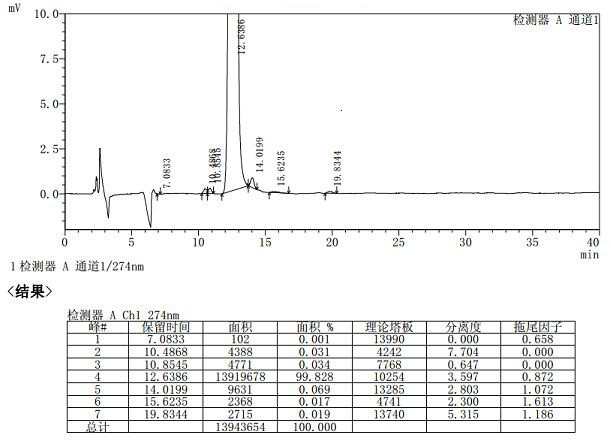
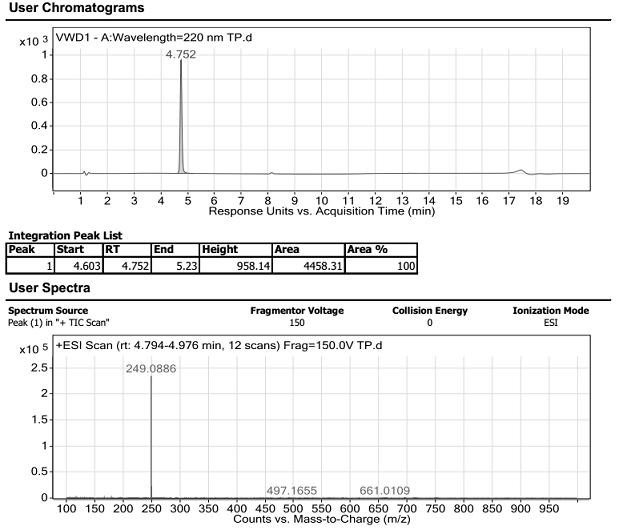
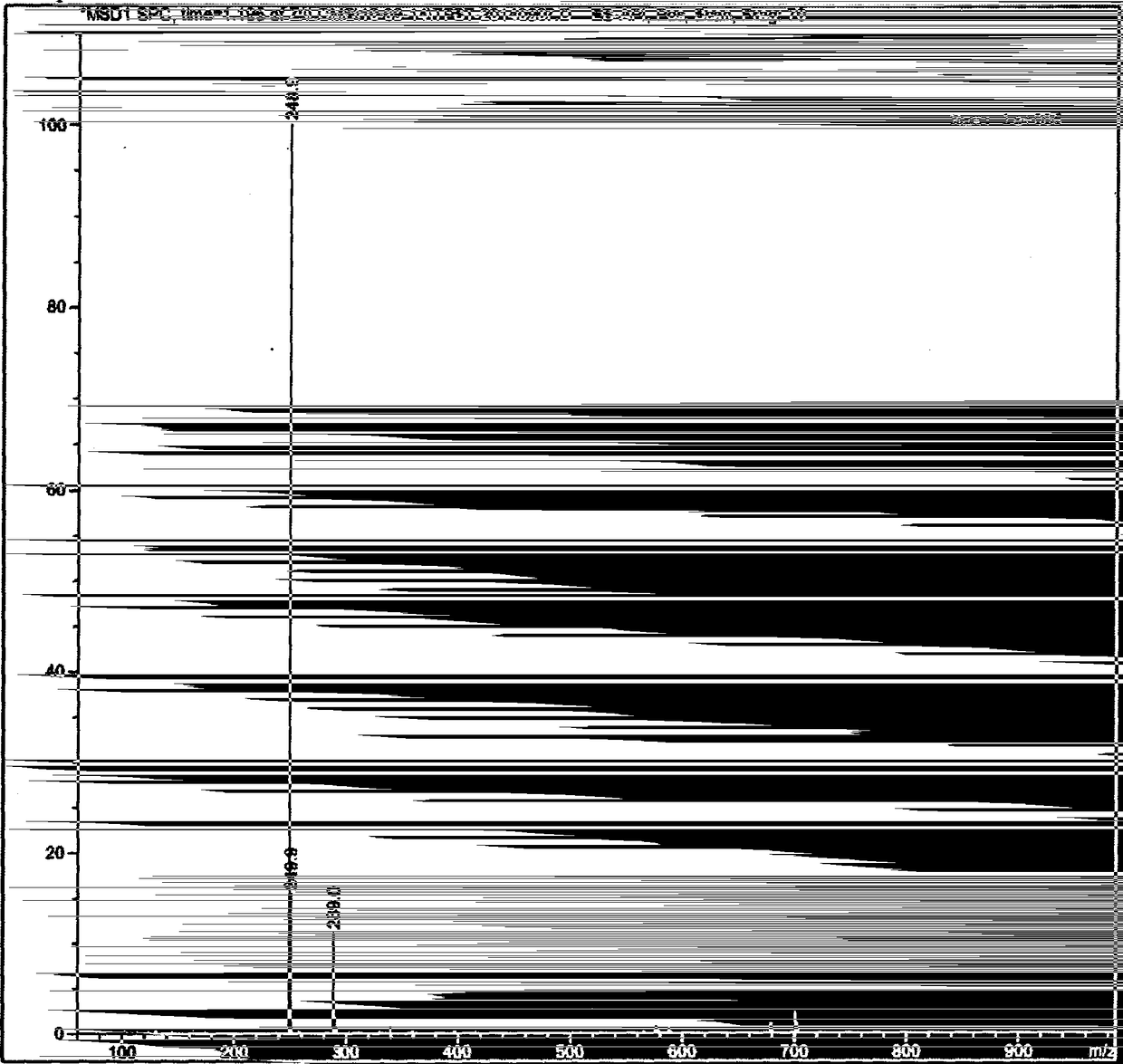
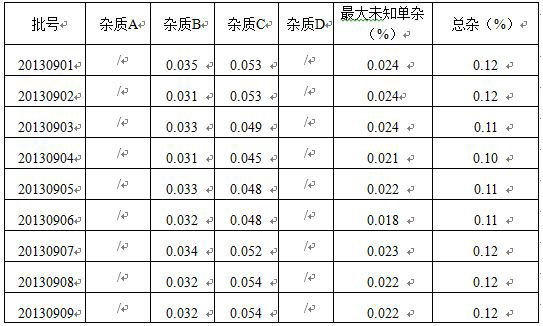
Method for determining related substances of new drug topiroxostat tablet
InactiveCN112485359AGood reproducibilityHigh precisionComponent separationNew medicationsFluid phase
The invention discloses a method for determining related substances of a new drug topiroxostat tablet, which uses high performance liquid chromatography to determine the related substances of the newdrug topiroxostat tablet to improve the reproducibility and precision so as to better control the impurities of the new drug topiroxostat tablet.
Owner:CP PHARMA QINGDAO CO LTD +1
Synthesis method of topiroxostat
The invention belongs to the field of medicine and chemical industry and discloses a synthesis method of topiroxostat. The method comprises that 4-cyanopyridine as a starting raw material is oxidized by hydrogen peroxide to form an intermediate 1, the intermediate 1, sodium methoxide and ammonium chloride undergo a reaction to produce an intermediate 2, the intermediate 2 and 4-cyanopyridine undergo an annulation reaction under action of cuprous bromide and sodium carbonate to produce an intermediate 3, and the intermediate 3 and trimethylsilyl cyanide undergo a cyanation reaction to produce topiroxostat. The method utilizes low-price 4-cyanopyridine as a starting raw material, and in the first reaction step, methanol is used as a solvent and 4-cyanopyridine nitrogen oxide as the intermediate 1 is prepared. The method has a high yield and produces a high-purity product. The whole route is easy to operate and is conducive to industrial mass production.
Owner:KAIFENG PHARMA GRP +2
A kind of synthetic method of topicastat
Owner:KAIFENG PHARMA GRP +2
Preparation method of topiroxostat
The invention provides a preparation method of topiroxostat, and relates to the technical field of medicine synthesis. The preparation method of the topiroxostat comprises the following steps: carrying out heat-preservation stirring reaction on 4-cyanopyridine and hydrazine hydrate in the presence of a solvent and an alkaline reagent to obtain an intermediate 1; carrying out heating stirring reaction on 4-picolinic acid in the presence of an acidic reagent and hydrogen peroxide to obtain isonicotinic acid nitrogen oxide; carrying out heat-preservation stirring reaction on the intermediate 1 and isonicotinic acid nitrogen oxide under the catalysis conditions of a solvent and a condensing agent to obtain an intermediate 3; carrying out heating stirring reaction on the intermediate 3 in the presence of a solvent and cyanide under the protection of nitrogen to obtain an intermediate 4; and carrying out reflux reaction on the intermediate 4 in the presence of an acidic reagent, cooling to room temperature, and filtering to obtain a topiroxostat solid. According to the invention, the topiroxostat is obtained through hydrazinolysis, oxidation, condensation, cyanidation and cyclization, isonicotinic acid and 4-cyanopyridine are selected as starting materials, the preparation method is low in production cost, high in yield, high in purity and few in three wastes, and the preparation method is suitable for industrial production of the topiroxostat and the intermediate thereof.
Owner:南京安一合医药科技有限公司
Orally disintegrating tablet containing topiroxostat and preparation method of orally disintegrating tablet
InactiveCN106880603AImprove solubilityImprove insoluble propertiesOrganic active ingredientsSkeletal disorderOrally disintegrating tabletAdhesive
The invention discloses an orally disintegrating tablet containing topiroxostat and a preparation method of the orally disintegrating tablet, belonging to the technical field of medicines. The orally disintegrating tablet contains opiroxostat, a hydrotropic filler, a water-soluble adhesive, a disintegrating agent, a lubricant, a corrigent, and the like, the preparation process is simple, the taking is convenient, the effects are rapid, the time for reaching the peak is short, and the curative effects are obvious.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
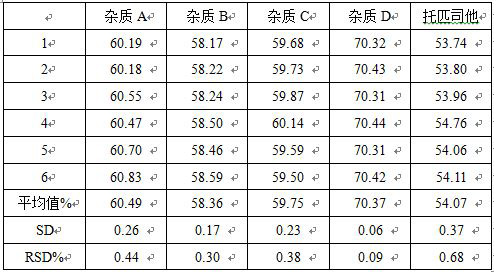
Method for detecting content of new drug topiroxostat tablet for treating gout
InactiveCN112394128AAvoid product qualityGood reproducibilityComponent separationNew medicationsFluid phase
The invention discloses a method for detecting the content of a new drug topiroxostat tablet for treating gout, which uses high performance liquid chromatography to determine the content of the new drug topiroxostat tablet, can improve the reproducibility, and enables the detection result to be more reliable, and thereby the product quality of the new drug topiroxostat tablet is better controlled.
Owner:CP PHARMA QINGDAO CO LTD
Topiroxostat controlled-release tablet and preparation method thereof
InactiveCN105434389AAvoid side effectsReach plasma concentrationOrganic active ingredientsSkeletal disorderSide effectFoaming agent
The invention discloses a topiroxostat controlled-release tablet and a preparation method thereof. The topiroxostat controlled-release tablet is composed of a drug-containing tablet core and a coating membrane layer covering the drug-containing tablet core, the drug-containing tablet core comprises topiroxostat, a filling agent and sodium chloride, and the coating membrane layer comprises a controlled-release material, plasticizer and a pore-foaming agent. During preparation, the drug-containing tablet core is wrapped with the coating membrane layer, and the topiroxostat controlled-release tablet is obtained. The topiroxostat controlled-release tablet and the preparation method thereof are characterized in that the topiroxostat controlled-release tablet is composed of a quick-release part and a controlled-release part; after the topiroxostat controlled-release tablet enters a human body, the quick-release part is released rapidly, a certain plasma concentration is achieved, the controlled-release part is released slowly, the certain plasma concentration is maintained, good drug efficacy can be achieved, and the side effect of the drug can be effectively avoided. The topiroxostat controlled-release tablet has the advantages of being rapid to dissolve, rapid to absorb, high in bioavailability, good in stability, convenient to take and the like, and therefore the side effect brought by taking the drug to a patient is reduced. The preparation technology is simple, the obtained product is stable in quality, and the preparation method is suitable for large-scale production.
Owner:CP PHARMA QINGDAO CO LTD
Topiroxostat tablet and preparation method thereof
ActiveCN112516094AIncrease dissolution rateImprove bioavailabilityOrganic active ingredientsSkeletal disorderTopiroxostatBioavailability
The invention relates to a topiroxostat tablet. The topiroxostat tablet is prepared by the following steps: grinding effective components and a carrier material through a ball mill to prepare a topiroxostat pharmaceutical composition, carrying out wet granulation, and carrying out tabletting. An effective component in the tablet is topiroxostat, and the tablet comprises the following materials inpercentages by weight: 30% of topiroxostat, 40-68.5% of a carrier material, 2-4% of an adhesive, 2-20% of a disintegrating agent and 0.5-4% of a lubricating agent, wherein the carrier material is composed of a filler and a pH regulator. According to the topiroxostat tablet and the preparation method thereof, tartaric acid or citric acid with proper dosage is added as a pH regulator, and ball milling is carried out at specific grinding time, so that the prepared topiroxostat tablet is high in dissolution rate and high in bioavailability, topiroxostat is limited to be in a crystal form I ratherthan a mixed crystal form, and the stability of the raw materials is ensured.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
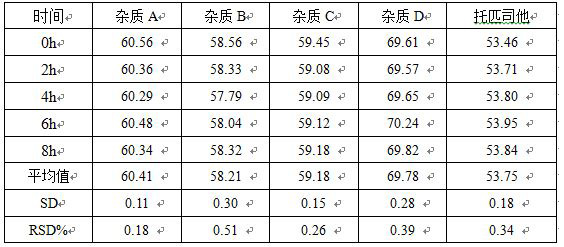
Detection method of topiroxostat tablets
InactiveCN111707765AGood linear relationshipImprove accuracyComponent separationPharmaceutical drugTopiroxostat
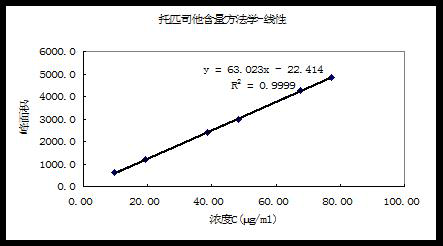
The invention relates to a detection method of topiroxostat tablets, and belongs to the field of pharmaceutical analysis. According to the detection method of topiroxostat tablets, topiroxostat is detected through an HPLC method. According to the detection method disclosed by the invention, the topiroxostat is good in linear relationship, good in accuracy and precision, strong in specificity and high in stability. The detection method is good in reproducibility, can meet the detection requirements of topiroxostat bulk drugs, and can be used for quality control of topiroxostat tablets.
Owner:CP PHARMA QINGDAO CO LTD +1
Refining method of topiroxostat
The invention belongs to the field of drug synthesis and relates to a method for refining high-purity topirastat. The refining method of topirastat according to the present invention comprises the following steps: adding topirastat crude product into an organic solvent, heating and stirring until completely dissolved, activated carbon decolorization, natural cooling and crystallization, filtering and drying to obtain high-purity topirastat Stata. The refined method of the invention has simple process, convenient operation, low production cost, high product purity, stable process and is suitable for industrialized production.
Owner:TIANJIN HANRUI PHARMA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile](https://images-eureka.patsnap.com/patent_img/55e48882-21a1-424c-b066-a4def80b3d6e/BDA0000560558790000021.PNG)
![Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile](https://images-eureka.patsnap.com/patent_img/55e48882-21a1-424c-b066-a4def80b3d6e/BDA0000560558790000022.PNG)
![Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile](https://images-eureka.patsnap.com/patent_img/55e48882-21a1-424c-b066-a4def80b3d6e/BDA0000560558790000023.PNG)