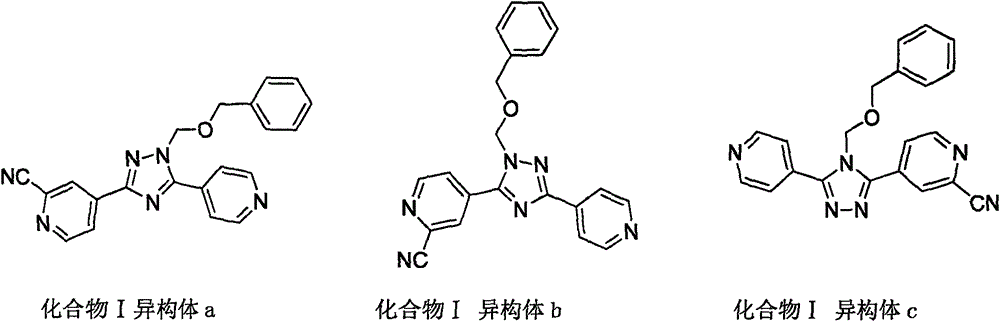
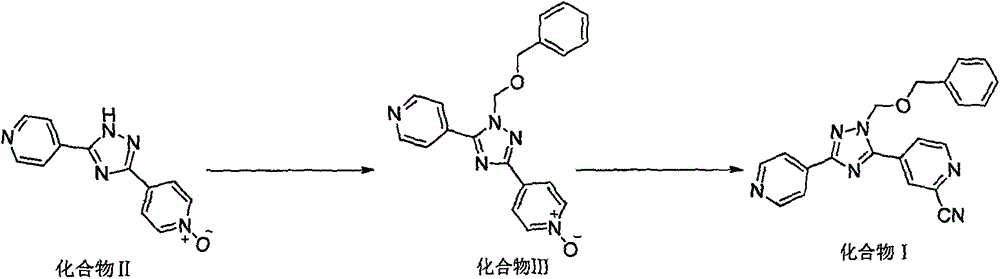
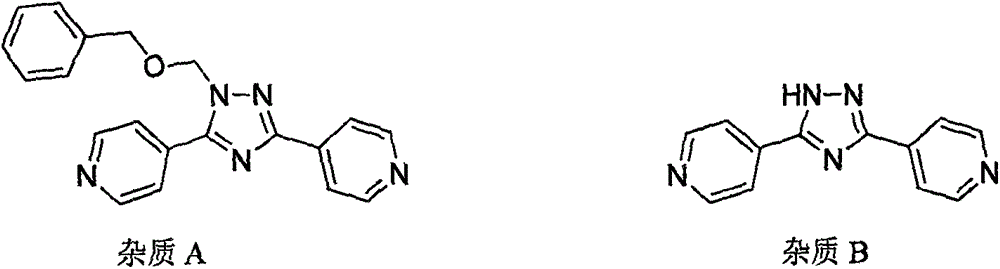
Preparation method of high-purity topiroxostat
A technology of topinostat and mixed solvents, which is applied in the field of preparation of topinostat, and can solve the problems of inability to obtain medicinal topinostat and difficult removal by processing technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the preparation of compound I crude product
[0037] Repeat patent EP1650204A1.
[0038] Add 600.0mL of N,N-dimethylformamide and 60.0g of compound II to the reaction flask in sequence, under the protection of nitrogen, cool down to 10°C, then add 39.9mL of benzyl chloride methyl ether and 40.2mL of triethylamine dropwise. After dropping, the temperature of the reaction solution was raised to 85°C, stirred for 2 hours, the reaction was stopped, the temperature was lowered to 0°C, 40.1mL of trimethylsilyl cyanide was added dropwise, the dropwise addition was completed, and stirred for 30min, the system turned into an orange suspension, and the temperature was controlled for 5 Below ℃, add 55.5mL of N,N,-dimethylcarbamoyl chloride dropwise, stir at 5°C for 1 hour, raise the temperature to 35°C, and stir for 10 hours, the system is dark brown with crystals precipitated. Cool the reaction solution to 5°C, slowly add 240mL of saturated aqueous sodium bicarbona...
Embodiment 2
[0040] Embodiment 2, the purification of compound I
[0041] Add 5.0g (content: 58%) of dark brown oil (Intermediate I) into the reaction flask, add 50mL of methanol, heat up to reflux, stir to dissolve, slowly add 15mL of purified water dropwise, and drop it over in 15 minutes. Stir for 40 minutes after dropping, cool down to room temperature, filter, and rinse the filter cake with 10 mL of methanol and water (1:3) and with 10 mL of purified water. After drying at 60°C, 2.4 g of off-white solid was obtained. Product purity: 94.1%; reduced yield: 82.7%. Topirestat was prepared in this way, and the obtained topicastat HPLC detection results were as follows, purity: 99.87%; impurity B: 0.04%.
Embodiment 3
[0042] The purification of embodiment 3 compound I
[0043] Add 5.0g (content: 58%) oily substance into the reaction flask, add 50mL of isopropanol, heat up to reflux, stir to dissolve, slowly add 15mL of purified water dropwise, and finish dropping in 15 minutes. Stir for 40 minutes after dropping, cool down to room temperature, filter, and rinse the filter cake with 10 mL of isopropanol and 10 mL of purified water. The material was dried at 60°C to obtain 2.5 g of solid. Purity: 94.8%; Yield after discount: 86.2%. Topirestat was prepared in this way, and the obtained topicastat HPLC detection results were as follows, purity: 99.65%; impurity B: 0.14%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com