Topiroxostat controlled-release tablet and preparation method thereof
A technology of topinostat and controlled-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve problems such as obvious side effects, achieve stable blood drug concentration, reduce Small irritation, the effect of reducing the peak and valley phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
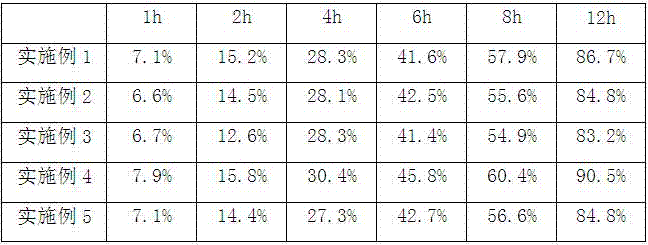
Examples
Embodiment 1
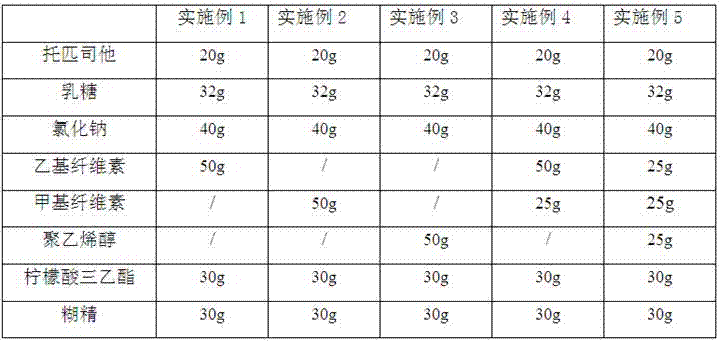
[0025] Prescription 1 (according to 1000 tablets)
[0026] Topicastat 20g
[0027] Lactose 32g
[0029] Ethyl cellulose 50g
[0030] Triethyl citrate 30g
[0031] Dextrin 30g
[0032] Preparation method:
[0033] (1) Take topicastat, filler and osmotic pressure active substance and mix evenly, use 85% ethanol as binder, make soft material, granulate with 18~24 mesh sieve, compress after drying, obtain drug-containing tablet core ;
[0034] (2) Dissolving controlled release material, plasticizer and porogen with 80% ethanol to make controlled release coating solution;
[0035] (3) Evenly spray the prepared controlled-release coating solution on the surface of the drug-containing tablet core prepared in step (1), and obtain the controlled-release topicastat tablet after drying.
Embodiment 2
[0037] Prescription 2 (according to 1000 tablets)
[0038] Topicastat 20g
[0039] Lactose 32g
[0041] Methylcellulose 50g
[0042] Triethyl citrate 30g
[0043] Dextrin 30g
[0044] Preparation method is the same as embodiment 1.
Embodiment 3
[0046] Prescription 3 (according to 1000 tablets)
[0047] Topicastat 20g
[0048] Lactose 32g
[0049] Sodium chloride 40g
[0050] Polyvinyl alcohol 50g
[0051] Triethyl citrate 30g
[0052] Dextrin 30g
[0053] Preparation method is the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com