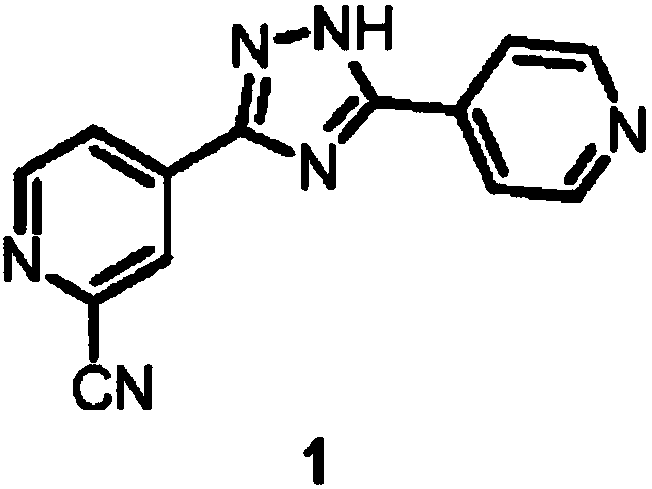
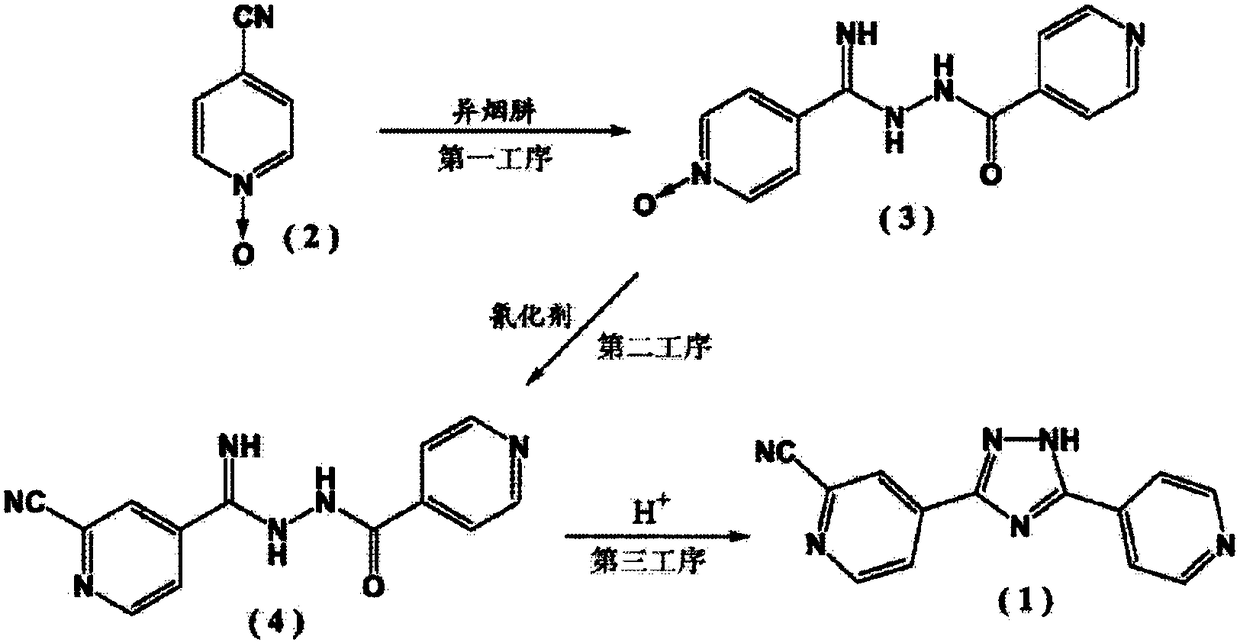
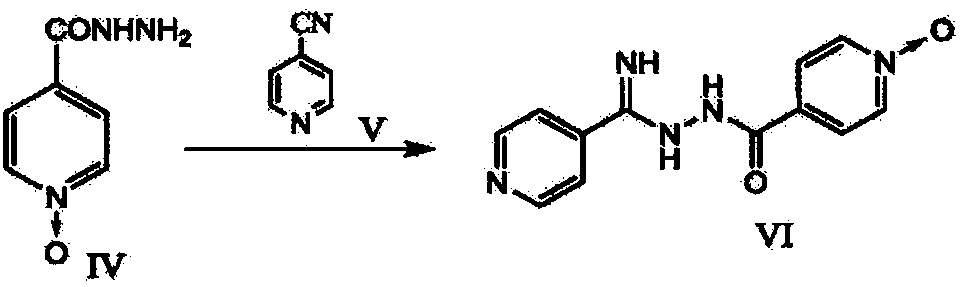
Topiroxostat and preparation method of intermediate of topiroxostat
A technology for an intermediate and topiramate, which is applied in the field of preparation of topiramate and its intermediates, can solve problems such as environmental protection problems and potential safety hazards, and achieve the effects of good industrial prospects, high yield and high product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment one, the synthesis of 4-pyridine carboximidic acid hydrazine
[0034]
[0035] Add 2.08kg of 4-cyanopyridine to a 20L reactor, add 10L of ethanol and 8.8g of sodium methoxide, raise the temperature to 40°C and stir for 9 hours, TLC detects that the basic reaction of the raw materials is complete; take another 20L reactor, add 5L of ethanol, 1.41kg 85% hydrazine hydrate and 12ml concentrated hydrochloric acid, pour the reaction solution from the previous step slowly, stir at room temperature for 1h, and TLC detects that the reaction of the raw materials is complete; 35°C rotary evaporation removes most of the solvent to a semi-oily solid, add 15L methyl The tert-butyl ether was stirred at room temperature, filtered, and vacuum-dried at 30° C. to obtain 2.31 kg of light yellow intermediate III, with a yield of 84.9%, and HPLC of 98%. [M+H] + = 137.2. 1 H NMR (400MHz, DMSO) δ: 8.50-8.52(d,2H), 7.63-7.65(d,2H), 5.74(br,2H), 5.40(br,2H).
Embodiment 2
[0036] Example two, the synthesis of 4-pyridinecarbohydrazide-N'-(2-cyanopyridine-4-carbonimido)
[0037]
[0038] Add 847g of compound IIB to a 20L reaction kettle, add 4.5L of DMF, then add 1.64Kg EDC·HCl and 1.14Kg HOBT, stir at room temperature to dissolve, then add 934g of intermediate III, stir at room temperature for 12h, TLC detection of raw materials The reaction was complete; 4.5 L of dissolved 719 g of sodium bicarbonate aqueous solution and 9 L of ethyl acetate were added, stirred at room temperature for 12 h, a large number of yellow solids precipitated, filtered, and air-dried at 50 ° C to obtain 1.38 kg of light yellow solid, yield 91.3%, HPLC 99.3 %. [M+H] + = 267.2. 1 H NMR (400MHz, MeOD) δ: 8.83-8.85(d,1H), 8.70-8.72(d,2H), 8.45(d,1H), 8.16-8.18(dd,1H), 7.87-7.89(dd,2H ).
Embodiment 3
[0039] Example three, the synthesis of 4-pyridinecarbohydrazide-N'-(2-cyanopyridine-4-carbonimido)
[0040]
[0041] Add 847g IIB to a 20L reaction kettle, add 4.5L DMF, then add 0.94Kg DIC and 1.14Kg HOBT, stir at room temperature to dissolve, then add 934g of intermediate III, stir at room temperature for 12 hours, and TLC detects that the raw materials have reacted completely; Add 4.5 L of water and 9 L of ethyl acetate, stir at room temperature for 2 h, a large amount of yellow solid precipitates, filter, and air-dry at 50°C to obtain 1.43 kg of light yellow solid, yield 94.7%, HPLC 99.0%. [M+H] + = 267.2. 1 H NMR (400MHz, MeOD) δ: 8.83-8.85(d,1H), 8.70-8.72(d,2H), 8.45(d,1H), 8.16-8.18(dd,1H), 7.87-7.89(dd,2H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com