Preparation method of topiroxostat
A technology of topinostat and solvent, which is applied in the field of drug synthesis, can solve the problems of difficult purification of topinostat crystals, low yield of cyano group substitution reaction, high production cost, etc., and achieve low production cost, cheap price, and three wastes little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
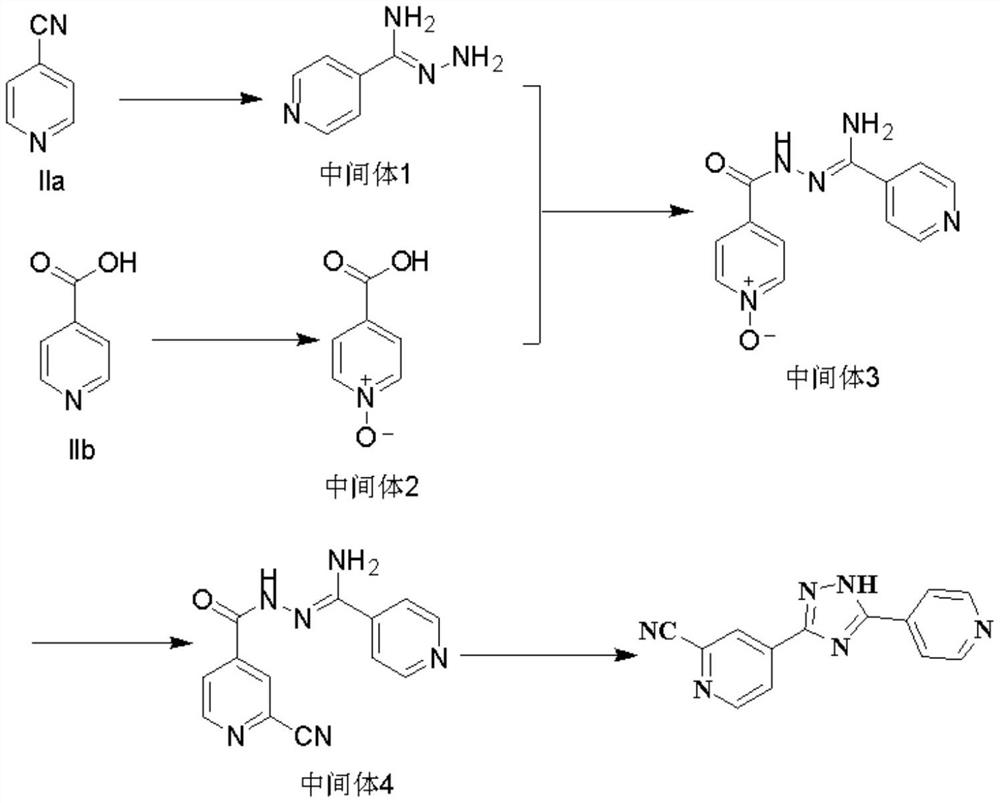
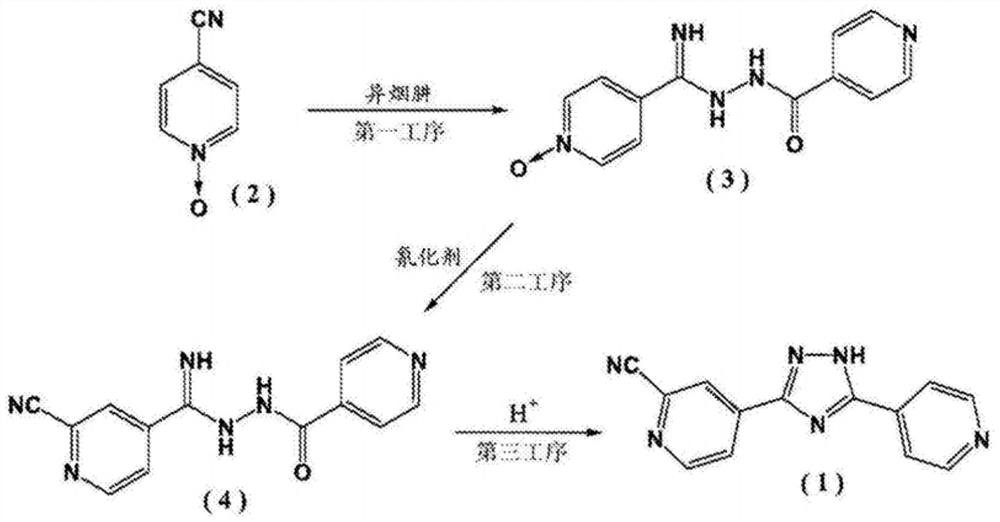
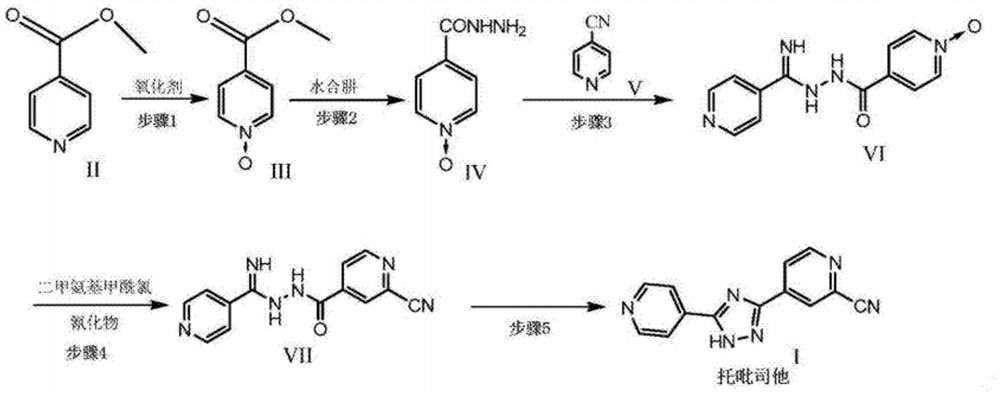
[0034] The present embodiment provides a kind of preparation method of topicastat, and chemical reaction formula is as follows:
[0035]
[0036] The preparation method of the present embodiment topicastat, comprises the following steps:
[0037] a. 4-cyanopyridine is reacted with 80% hydrazine hydrate under heat preservation and stirring for 2.5-4 hours in a solvent and an alkaline reagent, and the intermediate 1 is obtained after post-processing; wherein, the solvent is preferably ethanol, the alkaline reagent is sodium methoxide, and the holding temperature is 25±5°C, the post-treatment is to lower the temperature to 0-10°C, slowly add methyl tert-butyl ether dropwise, control the temperature at 5±5°C, stir and crystallize for 11-13 hours to obtain a yellow solid, filter, and use a small amount of Rinse with methyl tert-butyl ether, then vacuum dry at 25-35°C;
[0038] b. 4-Pyridinecarboxylic acid was heated and stirred under acidic reagent and 30% hydrogen peroxide for...
Embodiment 2
[0043] This embodiment provides a preparation method of intermediate 1, the chemical reaction formula is as follows:
[0044]
[0045] The specific operation process is as follows: Add 833 g (8 mol) of IIa4-cyanopyridine into a 50 L three-necked flask at room temperature, add 4.0 L of ethanol and 17 g (0.31 mol) of sodium methoxide, and stir at 25 ± 5 ° C for 3 h, thin layer Chromatographic detection showed that the raw materials were basically reacted completely; take another 10L four-neck flask, add 4.0L absolute ethanol and 303.6g (6.06mol) of 80% hydrazine hydrate, control the temperature at 25±5°C, keep stirring for 1-2h, thin The transition state reaction was detected by layer chromatography; the temperature was lowered to 0-10°C, and 24L methyl tert-butyl ether was slowly added dropwise, the temperature was controlled at 5±5°C, and the yellow solid was obtained by stirring and crystallizing for 12 hours, which was filtered, and the filter cake was washed with a small ...
Embodiment 3
[0048] This embodiment provides a kind of preparation method of intermediate 2, and chemical reaction formula is as follows:
[0049]
[0050] The specific operation process is as follows: add 210g (1.71mol) of IIb 4-pyridinecarboxylic acid into a 2L reaction flask, then add 560mL of acetic acid and 210mL of 30% hydrogen peroxide, heat up to 90°C for 3 hours, then add 210mL of hydrogen peroxide, and continue the reaction for 3 hours. The reaction of the raw material 4-pyridinecarboxylic acid was detected by thin-layer chromatography. Slowly cool down to room temperature and add 210mL of acetone, then cool down to 0-5°C and stir for crystallization for 4h, filter, rinse the filter cake with a small amount of methyl tert-butyl ether, Air blowing dried to obtain 213 g of white solid with a yield of 90% and a purity of 99%.
[0051] Mass Spectrometry [M+H] + =140.2, nuclear magnetic detection 1H NMR (400MHz, DMSO) δ: 13.553 (br, 1H), 8.285-8.303 (dd, 2H), 7.816-7.834 (dd, 2H)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com