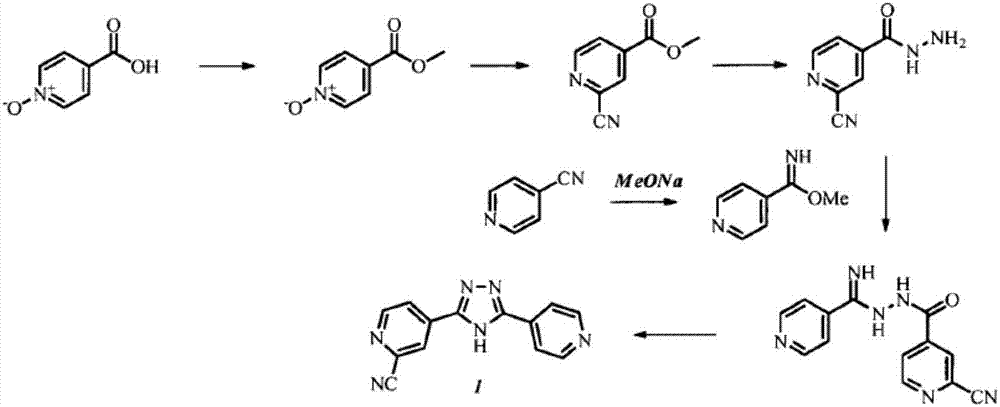
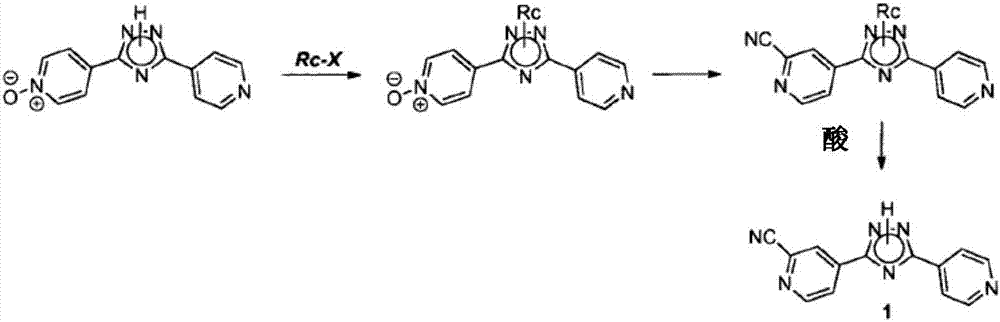
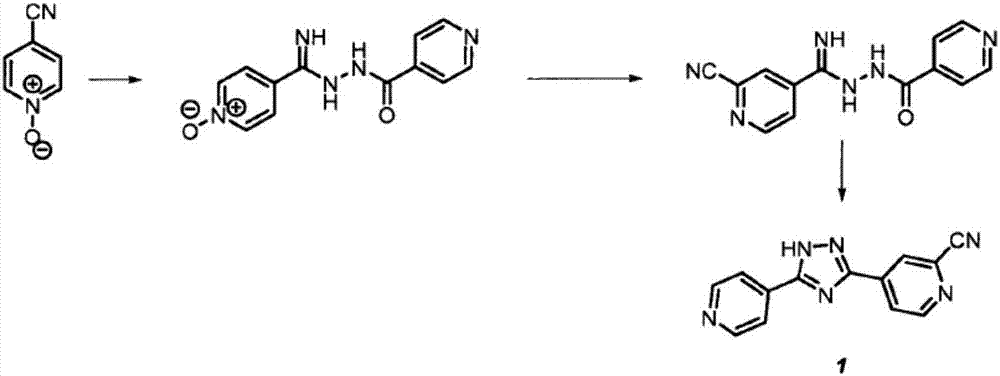
Methods for the preparation of topiroxostat and intermediates thereof
A technology for the application of topicastat, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, urinary system diseases, etc., and can solve problems such as expensive starting materials and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Preparation of 2-carbamoyl-4-cyano-pyridine 3a
[0079] 100 g of 4-cyano-pyridine followed by 800 ml of acetonitrile was added to a 3 L 4 neck round bottom flask equipped with a magnetic stirrer, thermometer and 500 mL addition funnel. Sulfuric acid (20ml) was added dropwise at room temperature. The resulting suspension was heated at 60°C. 60 ml of formamide solution (200 ml) in demineralized water (D.M. water) was charged to the addition funnel and added over a period of 10 min at 60°C. The resulting clear solution was heated at 70°C. 328 g of ammonium peroxodisulfate were added portionwise to the solution, maintaining a temperature between 70-75°C. After the addition was complete, the reaction mixture was stirred at 75°C for about 1 hour. The reaction was monitored by TLC and upon completion, 1 L of demineralized water was added. The solvent was distilled off under vacuum. The warm suspension was filtered off. The wet cake was rinsed with 500 mL of demineralize...
Embodiment 2
[0082] Preparation of 4-(5-(pyridin-4-yl)-1 H-1,2,4-triazol-3-yl)pyridineamide 7
[0083] 115 g of 2-carbamoyl-4-cyano-pyridine (3a), followed by 1150 mL of methanol were added to a 2 L hydrogenator equipped with a magnetic bar. 26.6 g of sodium methoxide were added portionwise at room temperature. The suspension was left under stirring for 2 hours to form the corresponding imino ether. After consumption of the starting material, 106.9 g of isoniazid (5b) were added. The resulting suspension was heated at 90° C. for 2 hours under pressure (4 bar). The reaction was heated at 100° C. for 6-10 hours with stirring and under pressure (4 bar). The suspension was filtered off, the wet cake was rinsed with 200 ml of methanol and dried under vacuum to afford 121 g of 4-(5-(pyridin-4-yl)-1H-1,2,4-triazol-3-yl)pyridineamide 7.
[0084] The filtrate was evaporated to dryness to afford 185 g of crude product. The crude product was dissolved in 555 ml demineralized water. The resulti...
Embodiment 3
[0087] Preparation of topinostat
[0088]10.0 g of 4-(5-(pyridin-4-yl)-lH-l,2,4-triazol-3-yl)pyridineamide (7) dissolved in 60 ml of THF was added to a device equipped with a magnetic stick and thermometer 250ml 3 neck round bottom flask. 7.86 ml of triethylamine were added. The reaction mixture was cooled at 0 °C and 6.95 ml trifluoroacetic anhydride (TFAA) was added dropwise. After addition of TFAA, the reaction mixture was left to stir at room temperature. The reaction was monitored by TLC and upon completion the solvent was evaporated and the crude API TFA salt was isolated as a yellow solid. The salt was dissolved in 50 ml MeOH and heated at 70 °C for 1 h. The suspension was cooled at room temperature and filtered off. The wet cake was rinsed with 200 ml of refrigerated methanol and dried under vacuum to provide 7.6 g of 4-(5-(pyridin-4-yl)-1H-1,2,4-triazol-3-yl)pyridine-2- Picolinitrile TFA salt.
[0089] 1H-NMR (500MHz, DMSO) δ8.94(d, 1H), 8.89(d, 2H), 8.54(s, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com