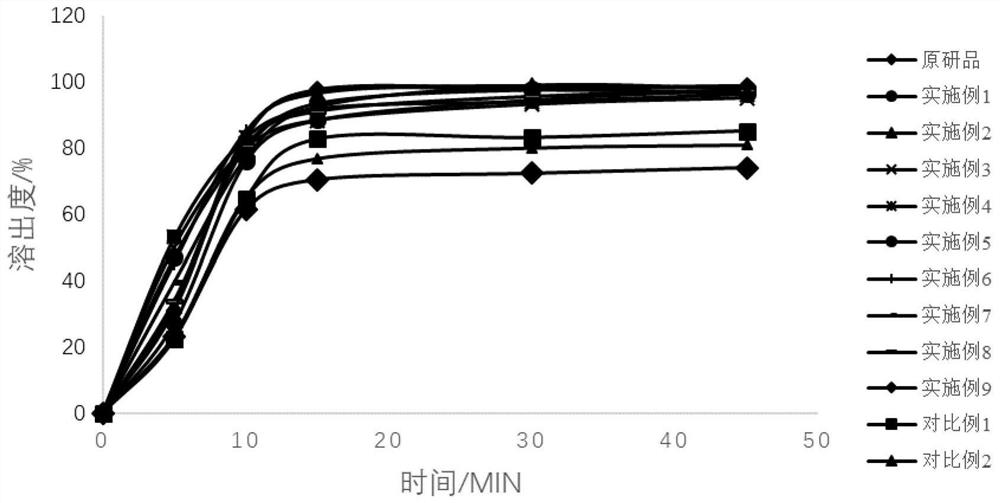
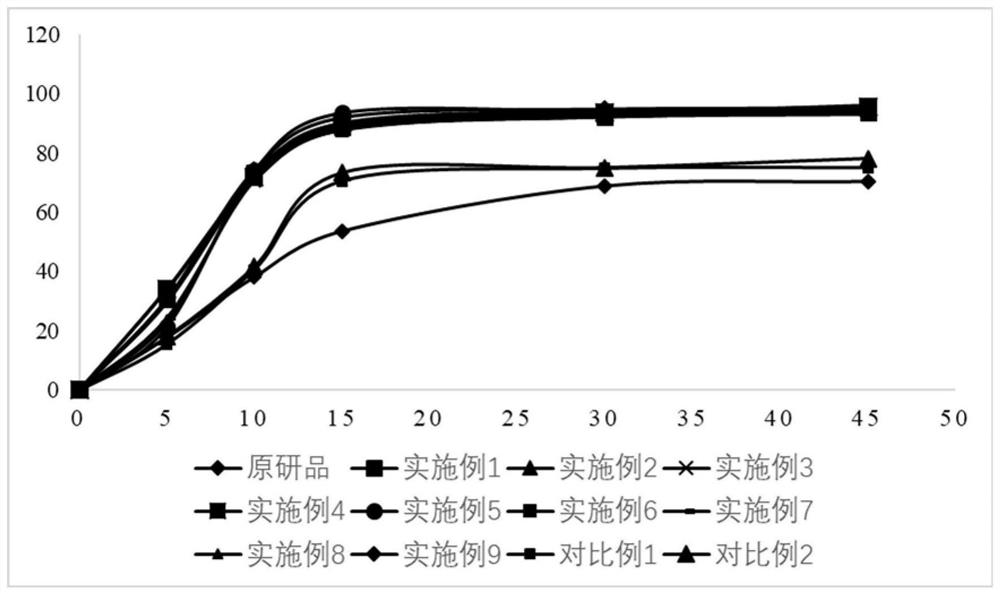
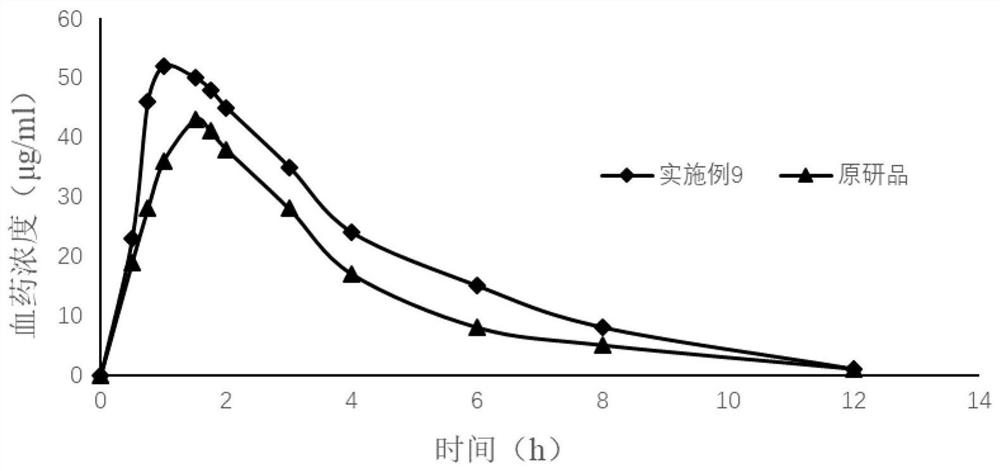
Topiroxostat tablet and preparation method thereof
A technology of topinostat and sita tablets, which is applied in the field of pharmaceutical preparations, can solve problems such as emulsion breaking, poor bioavailability, and difficulty in dissolving, and achieve the effects of high dissolution rate, ensuring stability, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Prescription and Process of Tribute
[0036] When the prescription is seen, his specification is 60mg / piece, and the amount is 1000 pieces, and the components of each component are:
[0037] 1000 pieces Composition (G) Toris 60 lactose 60 Lactic acid 38 Patternone 8 corn starch 30 Micro-powder silica gel 2 Sodium fumarate 2 Total 200
[0038] The preparation method is shown in:
[0039] (1) Take the right tribe, lactose, the lactose is 60 mesh sieve, adding the ball mill, adding lactic acid, grinding 30 min, and obtaining a mixed powder;
[0040] (2) Mix the mixed powder with the prescription amount of polymeridone, corn starch, and purify the water granulation, the wet particles are fluidized and dried, and the dry particles are obtained.
[0041] (3) After mixing dry particles with the prescription amount of micro-powder silica gel, sodium stearomarate, the hardness is controlled to 6-10kg, and the...
Embodiment 2
[0042] Example 2 Prescription and Process of Torret
[0043] When the prescription is seen, his specification is 60mg / piece, and the amount is 1000 pieces, and the components of each component are:
[0044] 1000 pieces Composition (G) Toris 60 lactose 42 Microcrystalline cellulose 42 tartaric acid 36 Hydroxypropylene 8 Sodium carboxymethyl starch 8 Magnesium stearate 4 Total 200
[0045] The method for preparing the filling agent, a pH conditioner, a step (2) in step (1), a lubricant, a binder, step (3) in step (1), in Example 2 The prescription component corresponds to the replacement.
Embodiment 3
[0046] Example 3 Prescription and Process of Torret
[0047] When the prescription is seen, his specification is 60mg / piece, and the amount is 1000 pieces, and the components of each component are:
[0048] 1000 pieces Composition (G) Toris 60 Mannitol 40 Cyclodextrin 40 Sorbic acid 40 Hydroxypropylene 4 Low substitution hydroxypropylene 8 talcum powder 6 Sodium fumarate 2 Total 200
[0049] The preparation method is in Example 1, only the disintegrant, a pH regulator, a pH conditioner, a step (2) in step (1), a lubricant, a binder, step (3), according to Example 3 The prescription component corresponds to the replacement.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com