Method for detecting content of new drug topiroxostat tablet for treating gout
A technique of topinostat and a detection method, which is applied in the field of medicine, can solve problems such as obvious side effects, and achieve the effects of improving reproducibility, good control, and reliable test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
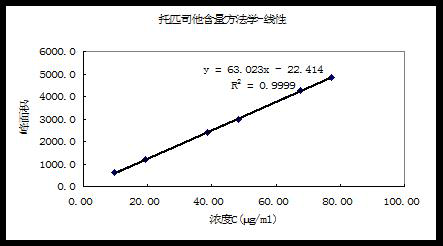
[0014] Example 1. Linear test
[0015] Chromatographic conditions:
[0016] Chromatographic column: Merck C18 (150×4.6mm×5μm)
[0017] Methanol: 20mmol / L potassium dihydrogen phosphate (adjust pH to 3.2 with phosphoric acid)-acetonitrile (80:20)
[0018] Detection wavelength: 220nm; flow rate: 1.0ml / min; injection volume: 10μl
[0019] Take an appropriate amount of the reference substance of this product, dissolve it with a diluent and quantitatively dilute it to make a solution containing about 0.5 mg of topirastat per 1 ml, as the test sample stock solution. Precisely measure 1ml of the test sample stock solution, put it in a 10ml volumetric flask, add methanol to dilute to the mark, shake well, and use it as the test solution for each concentration gradient. Precisely measure 10 μl of each test solution, inject it into a liquid chromatograph, and record the chromatogram. A linear regression was performed on the peak area A by the concentration C of the test solution, a...
Embodiment 2
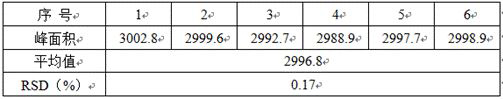
[0024] Example 2. Injection precision
[0025] Prepare the test solution with a concentration of 0.05mg / ml, inject 6 times continuously, record the chromatogram, and investigate the injection precision. The results are shown in Table 2:
[0026] Table 2 Injection precision test results
[0027]
[0028] The test results show that this method has good injection precision and is suitable for content determination.
Embodiment 3
[0029] Example 3. Sample solution stability
[0030] Take this product, dissolve it with methanol and dilute it into a test solution with a concentration of about 50μg / ml, inject the sample at 0, 2, 4, 6, and 8 hours respectively, record the peak area and calculate the average value and relative standard deviation. The results are shown in table 3:
[0031] Table 3 Sample solution stability
[0032]
[0033] The results showed that the test solution for the content determination of this product was stable within 8 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com