Method for determining related substances of new drug topiroxostat tablet
A technology of topicastat and related substances, applied in the field of medicine, can solve the problems of obvious side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]Example 1 Input-to-spectrum conditions
[0015]Columns: C18 (Merck C18, 150 × 4.6mm, 5 μm);
[0016]Detection wavelength: 270 nm, 220 nm; column temperature: 25 ° C; injection volume: 20μL
[0017]The mobile phase A is 0.02 mol / L dihydrogen potassium (adjusted pH to 3.2), and the mobile phase B is acetonitrile, and the linear gradient elution is performed in the table:
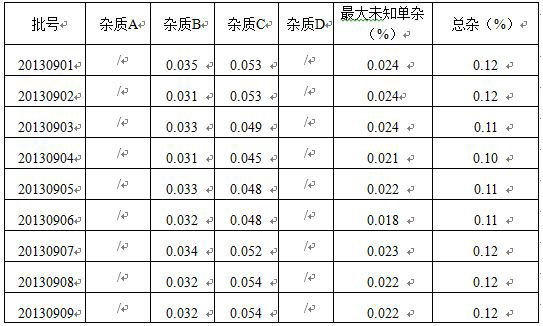
[0018]
[0019](1) Determination of the concentration of the solution of the substance
[0020]Take the right amount of this product, grind into fine powder, accurately weigh (about 25mg of tribe), set 50mL volumetric flask, add appropriate amount of methanol ultrasound to help, add methanol to the scale, shake, filter The filtrate is taken as a test solution, and 20 μl of the test solution is taken, and the liquid chromatography is injected into the liquid chromatography. The experimental results show that when the sample concentration is 0.5 mg / ml, it can meet the effective detection of impurities, and therefore, the conc...
Embodiment 2
[0023]Example 2 destructive test
[0024]Take the appropriate amount of powder fine, with high temperature, acid, alkali, oxidation, lighting, etc., for discrete conditions, to test the product, to examine whether the selected chromatographic conditions can detect the pallets. The resulting degradation product, the detection wavelength is 220 nm and 270 nm, the specifically as follows:
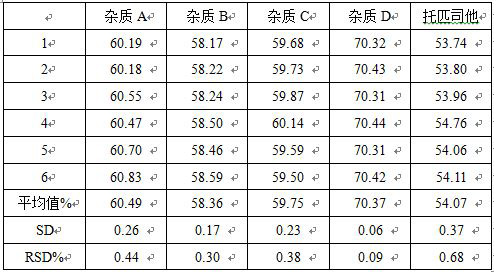
[0025]1 Unexpected: Take the right amount of this product (about 25mg of the tross), precision weighing, set 50mL volumetric flask, add methanol to dissolve and dilute to the scale, shake, filter,
[0026]2 high temperature damage (solid): Take the right amount of this product, then heating in an oven at 105 ° C for 24 hours, cool, precisely (about 25mg of Trib), set 50mL volumetric flask, add methanol ultrasound Dissolve and dilute to the scale, shake well, filter it,
[0027]3 high temperature damage (liquid): Take the right amount of this product fine powder, precision weighing (about 25mg of the Cheter), se...
Embodiment 3
[0033]Example 3 Stability test of the test solution
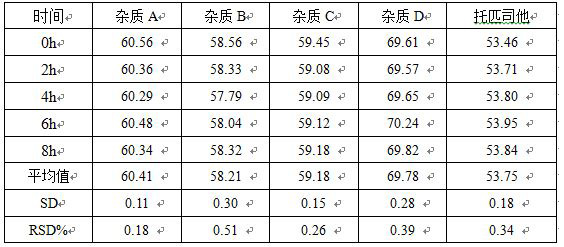
[0034]Take the right amount of this product fine powder, add methanol, ultrasonic dissolved and dilute to prepare a solution containing 0.5 mg of the tribe in each 1 ml as the test solution. The post-sample analysis of 0H, 2H, 4H, 6H, 8H, and the stability of the sample solution was found, and the results were shown in Table 1.
[0035]Table 1 Stability test results of the sample solution
[0036]
[0037]The test results show that the product sample solution is placed in 8 hours within 8 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com