Preparation method of topiroxostat
A technology of topinostat and intermediates, which is applied in the field of preparation of pharmaceutical raw materials, can solve the problems of low yield, poor safety, and many by-products, and achieve the effects of high product purity, low production cost, and less toxic substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
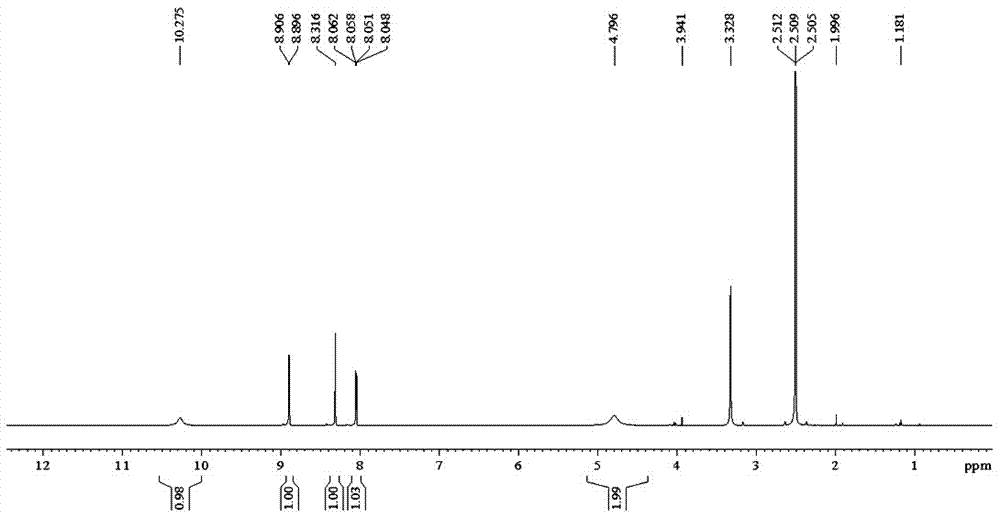
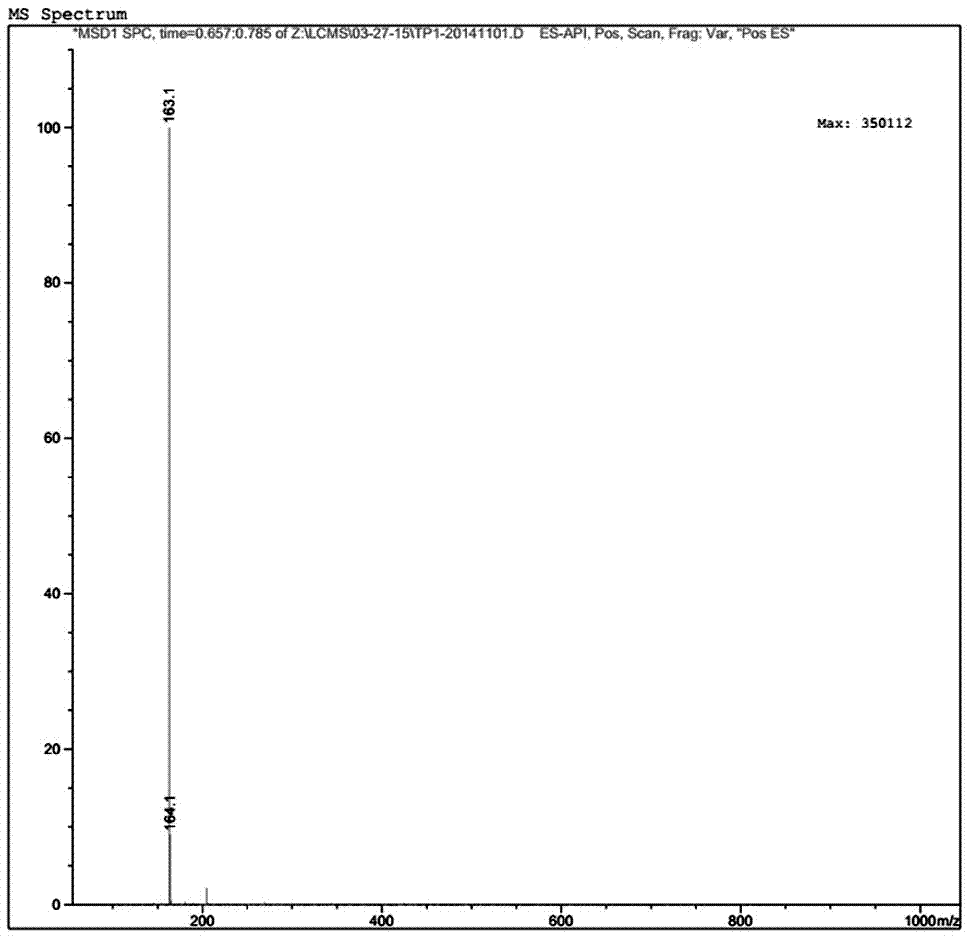
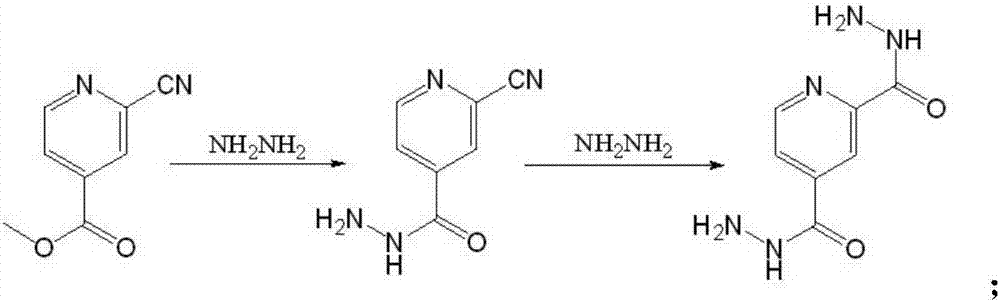
[0029] Add 20g of methyl 2-cyanoisonicotinate and 160ml of ethanol into a glass reaction bottle, stir and cool down to -15°C, start to add 21.6g of hydrazine hydrate dropwise while stirring, and control the temperature during the dropping process to about -15°C, about After 30 minutes of dripping, after the addition is completed, the temperature is controlled at -15°C and the reaction is stirred for 2.5 hours. After the end point of the reaction is monitored by TLC, the reaction is stopped, centrifuged, and the filter cake is collected. The filter cake is washed with 50ml of ethanol and dried. Chloromethane, add 260ml of hydrochloric acid aqueous solution with a volume concentration of 10% under stirring, stir to dissolve all the solids, separate the liquids, add 120ml of ethyl acetate to the water phase, adjust the pH to 6.5 with KOH under stirring, separate the liquids after stirring for 10 minutes, and collect For the organic phase, repeat the above extraction operation for ...
Embodiment 2
[0040] Put 100ml of absolute ethanol into a 500ml reaction bottle, add sodium sliced into thin slices, stir to prepare sodium ethoxide, continue to stir for 1h after the sodium is completely reacted, add 10.8g of 4-cyanopyridine, stir at 25°C for 1h, add Acetic acid aqueous solution, adjust the pH value to 5.0, stir for 10 minutes, add 14 g of the intermediate 2-cyanoisonicotinic acid carbohydrazide prepared in Example 1, start oil bath heating and circulation, reflux at 80 ° C, reflux for 7 hours, and monitor by HPLC , when the raw material 2-cyanoisonicotinic acid carboxylhydrazide obtained by the peak area normalization method is ≤0.5%, stop heating and circulation, release the heat transfer oil in the jacket, wait until the temperature is lowered to below 30°C, discharge the material and centrifuge, The filter cake was rinsed twice with 50ml ethanol. The filter cake was transferred to an enamel baking tray and dried under reduced pressure (80°C / <-0.9MPa / 8h) to obtain 18g...
Embodiment 3
[0054] Add 20g of methyl 2-cyanoisonicotinate and 160ml of ethanol into a glass reaction bottle, stir and cool down to -15°C, start to add 14.4g of hydrazine hydrate dropwise while stirring, and control the temperature during the dropping process to about -15°C, about After 30 minutes of dripping, after the addition is completed, the temperature is controlled at -15°C and the reaction is stirred for 2.5 hours. After the end point of the reaction is monitored by TLC, the reaction is stopped, centrifuged, and the filter cake is collected. The filter cake is washed with 50ml of ethanol and dried. Chloromethane, add 260ml of hydrochloric acid aqueous solution with a volume concentration of 10% under stirring, stir to dissolve all the solids, separate the liquids, add 120ml of ethyl acetate to the water phase, adjust the pH to 6.5 with KOH under stirring, separate the liquids after stirring for 10 minutes, and collect For the organic phase, repeat the above extraction operation for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com