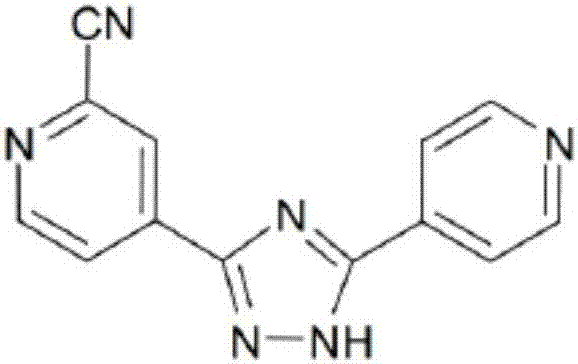
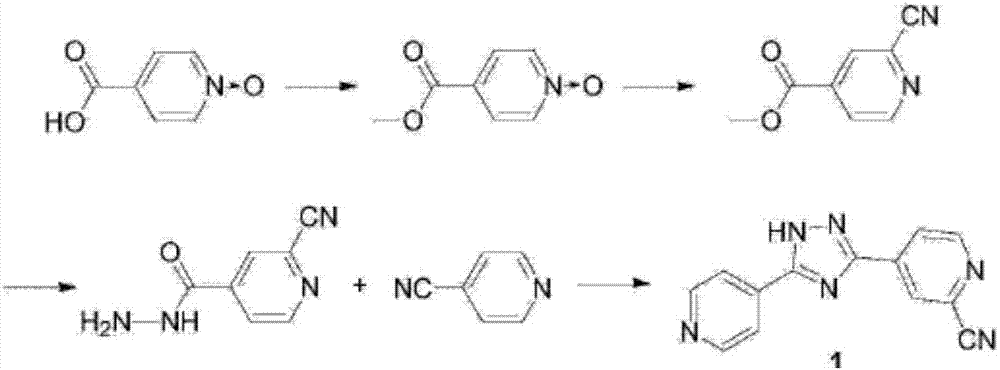
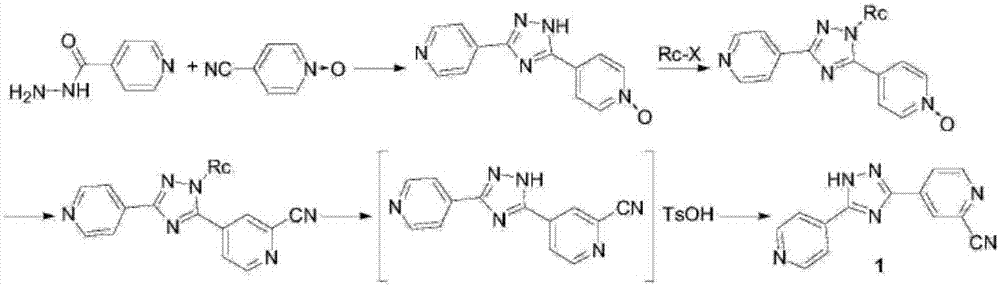
Synthesis method of topiroxostat
A technology of topicastat and its synthetic method, which is applied in the direction of organic chemistry, can solve the problems of unfavorable industrial production, cost reduction, high cost, etc., and achieve the effect of convenient operation, good purity and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 250ml three-necked flask, add 1g of tungsten powder to 10ml of 30% hydrogen peroxide by mass, and react for 20 minutes at 40-50°C, then add 100ml of methanol, 20g of 4-cyanopyridine, and 20ml of 30% hydrogen peroxide by mass, and raise the temperature to 60-65°C, react for 6h, after the reaction is completed, cool and crystallize naturally, wait for the temperature to cool to 25°C, continue to stir for 1h, filter with suction, and dry to obtain 20.5g of white crystals, namely intermediate 1, with a yield of 89.1%. HPLC purity (normalized method): 99.8%. NMR data related to Intermediate 1: H 1 NMR (400M Hz DMSO), 7.92(d, J=7.2,2H), 8.39(d, J=7.2,2H)
[0030] In a 250ml three-necked flask, add 0.4g sodium methoxide, 200ml methanol, 20g 4-cyanopyridine nitrogen oxide (intermediate 1), heat up to 40°C, react for 2h, add 17.8g ammonium chloride, react for 1h and then cool naturally Continue to stir for 0.5 h at 25°C, filter with suction, and dry to obtain 35.8 g of wh...
Embodiment 2
[0033] Embodiment 2: the preparation method of intermediate 1
[0034] In a 250ml three-necked flask, add 1g of tungsten powder to 10ml of 30% hydrogen peroxide by mass, and react for 20 minutes at 40-50°C, then add 100ml of water, 20g of 4-cyanopyridine, and 20ml of 30% hydrogen peroxide by mass, and heat up To 60-65°C, react for 6h, after the reaction is completed, cool and crystallize naturally, wait for the temperature to cool to 25°C, continue to stir for 1h, filter with suction, and dry to obtain 18.9g of white crystals, crude product, HPLC purity (normalization method) : 80.5%.
Embodiment 3
[0035] Embodiment 3: the preparation method of intermediate 1
[0036] In a 250ml three-necked flask, add 2.8g of tungsten powder to 20ml of 30% hydrogen peroxide by mass, and react for 0.5h at 40-50°C, then add 100ml of methanol, 20g of 4-cyanopyridine, and 20ml of 30% hydrogen peroxide in sequence , heated up to 60-65°C, reacted for 5h, after the reaction was completed, cooled and crystallized naturally, after the temperature was cooled to 25°C, continued to stir for 1h, filtered with suction, and dried to obtain 19.5g of white crystals, namely intermediate 1, with a yield of 84.4 %, HPLC purity (normalized method): 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com