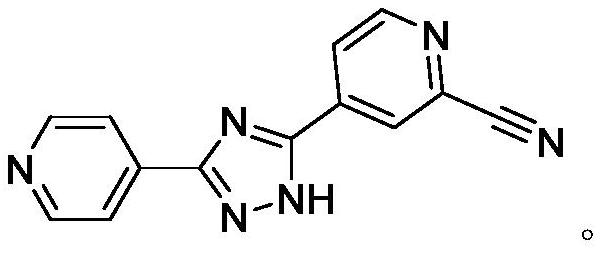
Preparation method of topiroxostat
A technology of topicastat and compounds, applied in the direction of organic chemistry and the like, can solve the problems of high cost of raw materials, harsh reaction conditions, long process routes, etc., and achieves the effects of high reaction yield, mild reaction, economical and environmentally friendly yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
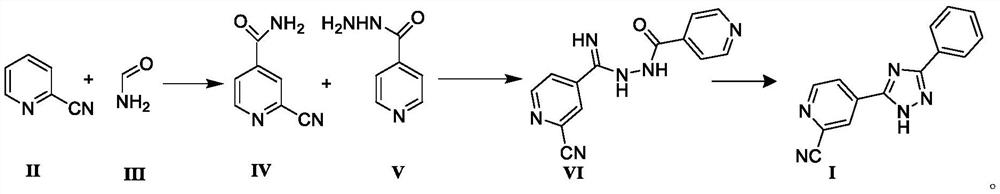
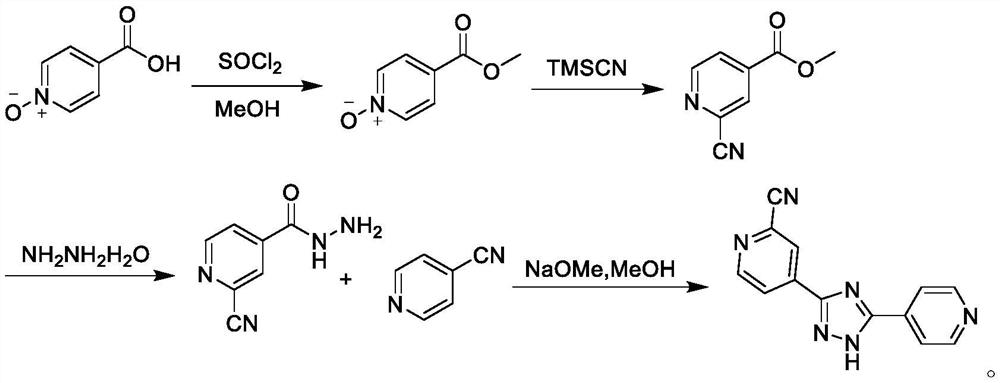
[0051] Preparation of Compound IV
[0052]Into a 5L three-neck flask, add 2-cyanopyridine (104.11g, 1.0mol) and 1000mL toluene at room temperature, stir and dissolve, then add concentrated sulfuric acid (39.23g, 0.4mol) dropwise, after dropping, heat up to 70℃~75℃ , add formamide aqueous solution (225.20g, 5.0mol) quickly, after dropping, start to add ammonium persulfate (342.30g, 1.5mol) saturated aqueous solution, after dropping, keep warm at 70-75°C, TLC monitors the reaction solution Add 1000mL of purified water to the mixture, cool to room temperature and crystallize for 2 hours, filter, wash the filter cake with purified water until neutral, then wash with 200mL of ethanol, and dry in vacuum for 12 hours to prepare 2-cyano-4-carbamoyl-pyridine , yield 98.7%, HPLC purity 99.88%.
Embodiment 2
[0054] Into a 5L three-neck flask, add 2-cyanopyridine (104.11g, 1.0mol) and 1000mL toluene at room temperature, stir and dissolve, then add concentrated sulfuric acid (39.23g, 0.4mol) dropwise, after dropping, heat up to 70℃~75℃ , add formamide aqueous solution (180.16g, 4.0mol) quickly, after dropping, start to add ammonium persulfate (342.30g, 1.5mol) saturated aqueous solution, after dropping, keep warm at 70-75°C for reaction, TLC monitors the reaction solution Add 1000mL of purified water to the mixture, cool to room temperature and crystallize for 2 hours, filter, wash the filter cake with purified water until neutral, then wash with 200mL of ethanol, and dry in vacuum for 12 hours to prepare 2-cyano-4-carbamoyl-pyridine , yield 94.2%, HPLC purity 99.79%.
Embodiment 3
[0056] Into a 5L three-necked flask, add 2-cyanopyridine (104.11g, 1.0mol) and 1000mL 1,2-dichloroethane at room temperature, stir and dissolve, then add hydrochloric acid (14.60g, 0.4mol) dropwise, dropwise, and heat up to 70 ℃ ~ 75 ℃, quickly add formamide aqueous solution (270.24g, 6.0mol), after dropping, start adding saturated aqueous solution of ammonium persulfate (342.30g, 1.5mol), after dropping, keep warm at 70 ~ 75 ℃, TLC After monitoring the completion of the reaction, add 1000 mL of purified water to the reaction liquid, cool to room temperature and crystallize for 2 hours, filter, wash the filter cake with purified water until neutral, then wash with 200 mL of ethanol, and dry in vacuum for 12 hours to prepare 2-cyano-4- Carbamoyl-pyridine, yield 93.6%, HPLC purity 99.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com