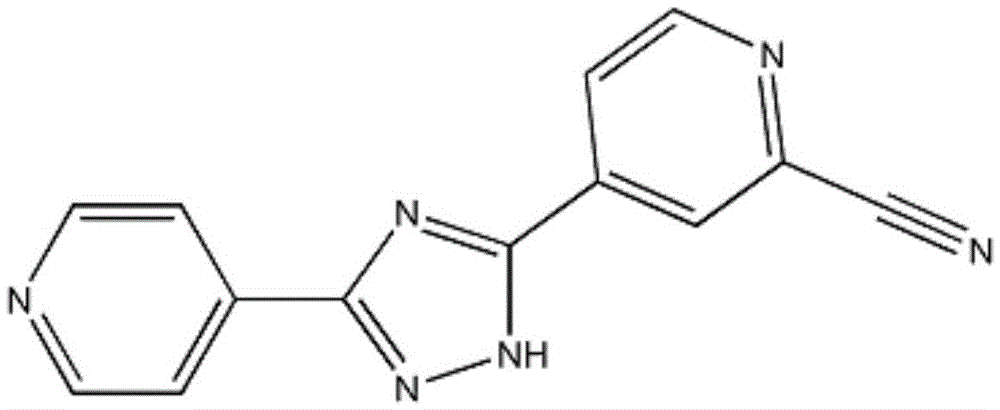
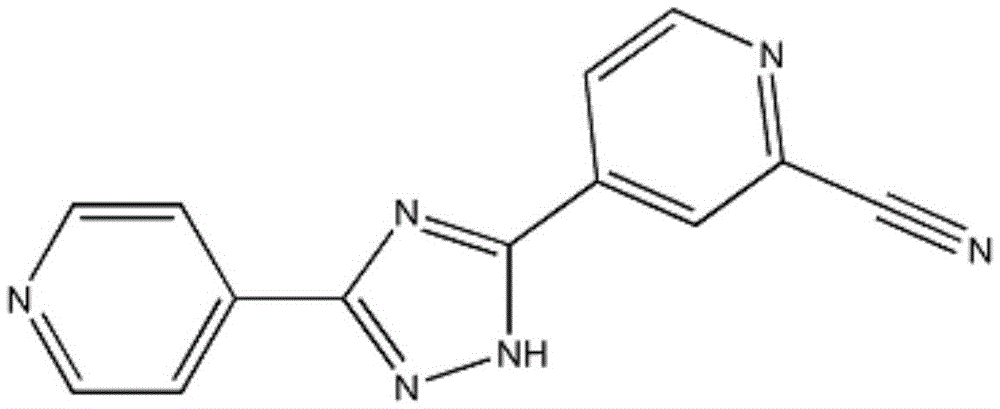
Orally disintegrating tablet containing topiroxostat and preparation method of orally disintegrating tablet
A technology of oral disintegrating tablet and topirastat, which is applied in the field of pharmaceutical preparations, can solve problems such as adverse reactions, and achieve the effects of enhancing solubility, fast onset of action and improving compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027]
[0028] Preparation Process:
[0029] (1) co-micronizing the topicastat and mannitol in proportion;
[0030] (2) Topicastat and mannitol, microcrystalline cellulose, sodium carboxymethyl starch are mixed homogeneously in mixer with prescription quantity;
[0031] (3) Add 4% hydroxypropyl cellulose aqueous solution to the above mixture to make wet soft material, and granulate;
[0032] (4) The above-mentioned granules are dried until the water content is less than 3%. After the granules are sized, the dosages of magnesium stearate, peppermint essence and aspartame are converted and mixed evenly with the dried granules;
[0033] (5) Measure the particle content after total blending, convert the tablet weight, and control the hardness to 3-5kgf for tableting. Prepared in 1000 tablets.
Embodiment 2
[0035]
[0036] Preparation Process:
[0037] (1) co-micronizing topinostat and lactose in proportion;
[0038] (2) the topicastat of prescription quantity is mixed with lactose, microcrystalline cellulose, sodium carboxymethyl starch in mixer;
[0039] (3) Add 4% hydroxypropyl cellulose aqueous solution to the above mixture to make wet soft material, and granulate;
[0040] (4) The above-mentioned granules are dried until the water content is less than 3%. After the granules are sized, the dosages of magnesium stearate, peppermint essence and aspartame are converted and mixed evenly with the dried granules;
[0041] (5) Measure the particle content after total blending, convert the tablet weight, and control the hardness to 3-5kgf for tableting. Prepared in 1000 tablets.
Embodiment 3
[0043]
[0044]
[0045] Preparation Process:
[0046] (1) co-micronizing topinostat and lactose in proportion;
[0047] (2) Topicastat and lactose, mannitol, croscarmellose sodium are mixed homogeneously in the blender of prescription quantity;
[0048] (3) adding 3% povidone aqueous solution to the above-mentioned mixture to make wet and soft materials, and granulating;
[0049] (4) The above-mentioned granules are dried until the water content is less than 3%. After the granules are sized, the dosages of magnesium stearate, peppermint essence and aspartame are converted and mixed evenly with the dried granules;
[0050] (5) Measure the particle content after total blending, convert the tablet weight, and control the hardness to 3-5kgf for tableting. Prepared in 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com