One-pot method for synthesizing Topiroxostat
A technology of topinostat and hydrazine hydrate, which is applied in the field of one-pot synthesis of topinostat, and can solve the cumbersome purification method of 2-cyanoisonicotinic acid methyl ester, cumbersome operation of topinostat, and limited industrial application, etc. problems, to achieve good production and practical value, high utilization rate of raw materials, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
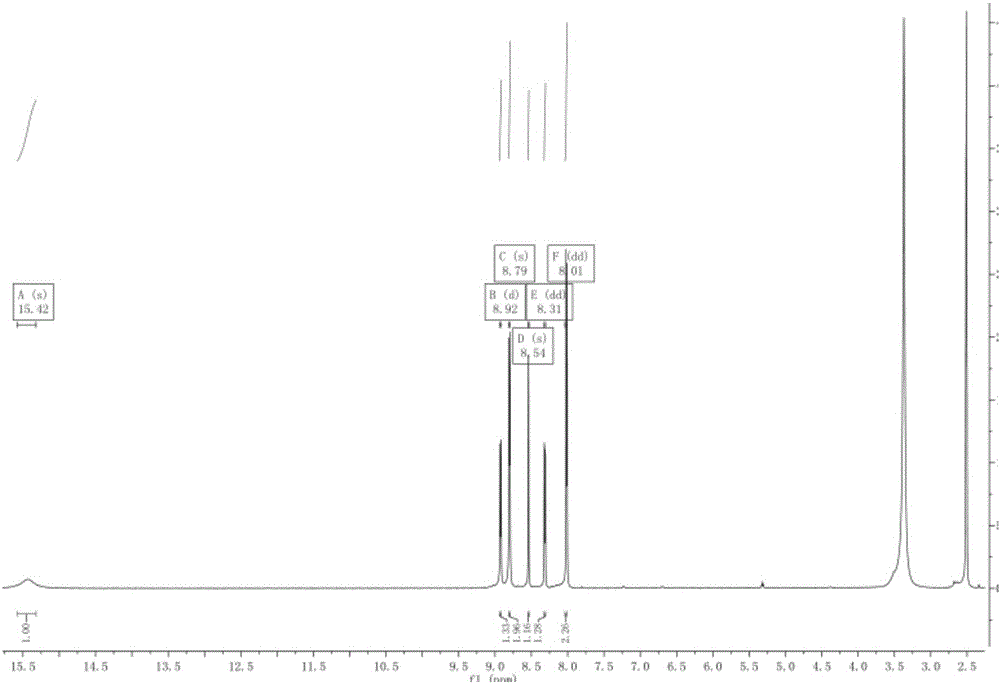
[0020] Add methyl 2-cyanoisonicotinate (487.1g, 3mol) and dissolve it in 1L ethanol in the reactor, stir at room temperature, add hydrazine hydrate (187.9g, 3mol) dropwise, then raise the temperature to 40°C and stir for 4h, TLC The reaction was detected (ethyl acetate:petroleum ether=1:2), and the reaction was completed. Then add sodium methoxide (28g, 0.5mol), stir for 20min until dissolved, then add compound 4-cyanopyridine (312.33g, 3mol), react at 80°C for 10h, and detect by TLC (ethyl acetate:petroleum ether=1:4) , after the reaction was finished, cooled to normal temperature for crystallization, the precipitated solid was filtered, washed with 500ml of ethanol, and vacuum-dried to obtain 405.5g of a light yellow powder product, topicastat, with a yield of 63%. mp: 323.5-325.7°C. ESI-MS(m / z):249[M+H] + . 1 H NMR (400MHz, DMSO) δ15.41(s, 1H), 8.92(d, J=5.1Hz, 1H), 8.79(d, J=5.9Hz, 2H), 8.53(s, 1H), 8.31(dd , J=5.1, 1.6Hz, 1H), 8.01(dd, J=4.5, 1.5Hz, 2H). The H-NMR spe...
Embodiment 2
[0022] Add methyl 2-cyanoisonicotinate (487.1g, 3mol) and dissolve it in 1L of methanol in a reactor, stir at 0°C, add hydrazine hydrate (187.9g, 3mol) dropwise, then raise the temperature to 10°C and stir for 1h, TLC The reaction was detected (ethyl acetate:petroleum ether=1:2), and the reaction was completed. Potassium carbonate (207g, 1.5mol) was added, stirred for 20min until dissolved, then compound 4-cyanopyridine (312.33g, 3mol) was added, reacted at 80°C for 2h, detected by TLC (ethyl acetate:petroleum ether=1:4) , after the reaction was finished, cooled to normal temperature and crystallized, the precipitated solid was filtered, washed with 500ml of methanol, and vacuum-dried to obtain 399.8g of light yellow powdery product topicastat, with a yield of 62.1%. mp: 323.8-326.7°C. ESI-MS(m / z):249[M+H] + . 1 H NMR (400MHz, DMSO) δ15.41(s, 1H), 8.92(d, J=5.1Hz, 1H), 8.79(d, J=5.9Hz, 2H), 8.53(s, 1H), 8.31(dd , J=5.1, 1.6Hz, 1H), 8.01 (dd, J=4.5, 1.5Hz, 2H).
Embodiment 3
[0024] Add methyl 2-cyanoisonicotinate (487.1g, 3mol) and dissolve it in 1L of ethyl acetate in a reactor, stir at room temperature, add hydrazine hydrate (187.9g, 3mol) dropwise, then raise the temperature to 30°C and stir for 2h , TLC detected the reaction (ethyl acetate:petroleum ether=1:2), and the reaction was complete. Then add sodium methoxide (28g, 0.5mol), stir for 20min until dissolved, then add compound 4-cyanopyridine (468g, 4.5mol), react at 60°C for 2h, TLC detection (ethyl acetate:petroleum ether=1:4) , after the reaction was finished, cooled to normal temperature for crystallization, the precipitated solid was filtered, washed with 500ml of ethyl acetate, and then vacuum-dried to obtain 385.5g of a light yellow powdery product, topicastat, with a yield of 59.8%. mp: 323.2-325.8°C. ESI-MS(m / z):249[M+H] + . 1 H NMR (400MHz, DMSO) δ15.41(s, 1H), 8.92(d, J=5.1Hz, 1H), 8.79(d, J=5.9Hz, 2H), 8.53(s, 1H), 8.31(dd , J=5.1, 1.6Hz, 1H), 8.01 (dd, J=4.5, 1.5Hz, 2H). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com