Preparation process and method for topiroxostat
A technology for the preparation of topinostat, which is applied in the field of preparation technology for topinostat, can solve problems such as unfavorable control of the quality of raw materials, high impurity content, and high toxicity of cyanide reagents, and achieve the goal of cyanohydrolysis The effect of increasing risk, improving product quality, and ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The invention discloses a preparation method of topicastat. Those skilled in the art can refer to the content of this article and make appropriate improvements to the process parameters. In particular, it should be pointed out that all similar replacements and modifications will be obvious to those skilled in the art, and they will all be deemed to be included within the scope of the present invention. The method and application of the present invention have been described through preferred embodiments, and it is obvious that relevant personnel can make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention. And apply the technology of the present invention.
[0051] Topinostat and [formula (IV), 4-pyridinecarbohydrazide-N'-(2-cyanopyridine-4-carbonimido)] detection method is the same, specifically as follows: chromatographic column is octadecyl Silane-...
Embodiment 1
[0055] Add 2.0L of methanol and 190g of 4-cyanopyridine into the reaction kettle, stir and dissolve at room temperature, add 5g of sodium methoxide, stir and react at a temperature of 20-30°C for 0.5 hours, cool down to 0-10°C and stir for 1 hour.
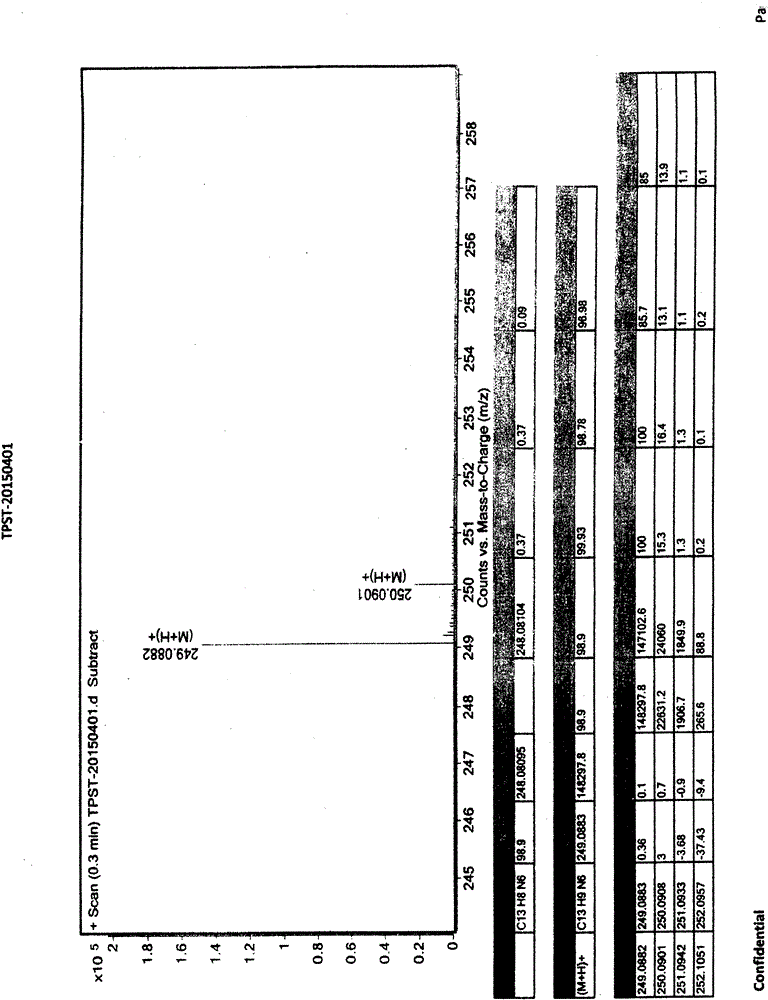
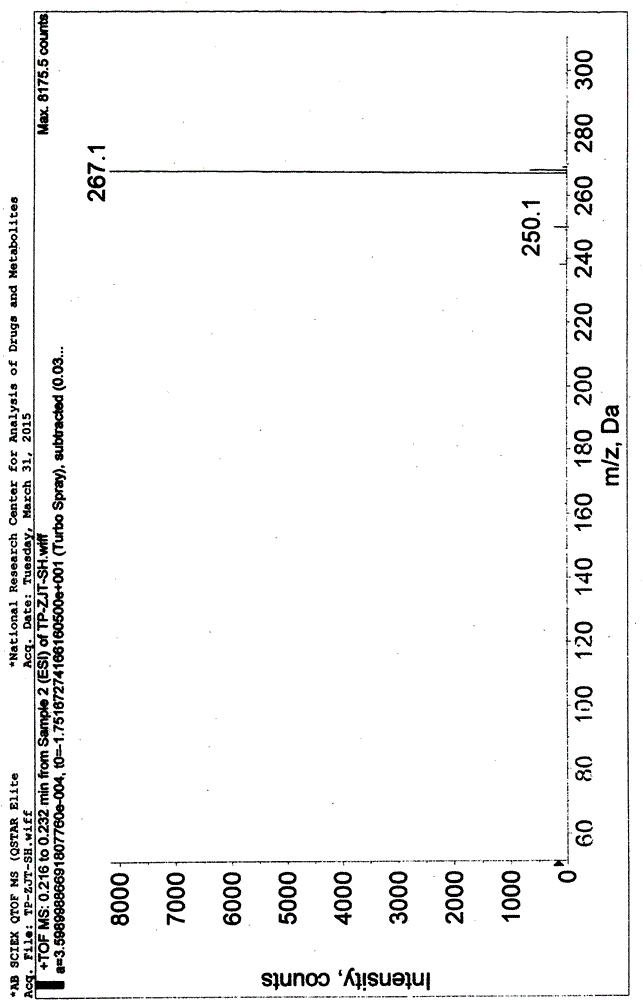
[0056] Add 280g of 2-cyanoisonicotinic acid hydrazide to the above reaction solution, keep warm at 0-10°C and stir for 4 hours. After the reaction is completed, filter and collect the filter cake, wash the filter cake with water, collect the filter cake, and dry the filter cake to obtain [Formula (IV), 4-pyridinecarbohydrazide-N'-(2-cyanopyridine-4-carbonimido)], weight 413g, yield 90%, off-white solid. 99.8% pure. Mass spectrum (ESI): 267.1 (M+1, molecular ion peak).
Embodiment 2
[0058] Add 2.0L of methanol and 190g of 4-cyanopyridine into the reaction kettle, stir and dissolve at room temperature, add 4g of sodium hydroxide, control the temperature at 30-40°C and stir for 0.5 hours, then cool down to 0-10°C and stir for 1 hour.
[0059] Add 280g of 2-cyanoisonicotinic acid hydrazide to the above reaction solution, keep it warm at 0-10°C and stir for 4 hours. After the reaction is complete, filter and collect the filter cake, wash the filter cake with phosphoric acid solution, collect the filter cake, and dry the filter cake to obtain [Formula (IV), 4-pyridinecarbohydrazide-N'-(2-cyanopyridine-4-carbonimido)], weight 391g, yield 85%, white solid. 99.7% purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com