Myricetin pharmaceutical eutectic crystal and preparation method thereof
A technology for myricetin and medicine, which is applied to the field of myricetin drug co-crystal and its preparation, can solve the problems of poor fat solubility, low bioavailability, limited application of myricetin, etc., and achieves wide application, improved dissolution rate and stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Accurately weigh 408.12mg of myricetin and 68.43mg of caffeine into 20mL transparent glass bottles, add 8mL of methanol, seal the glass bottles in a constant temperature water bath shaker, shake and react at 25°C for 12h; stop the reaction, filter, and take The filter cake is dried under a vacuum environment, and the product obtained is myricetin-caffeine eutectic.
Embodiment 2
[0057] Accurately weigh 525.13mg of myricetin and 129.13mg of caffeine into 20mL transparent glass bottles, add 10mL of methanol, seal the glass bottles in a constant temperature water bath shaker, shake and react at 25°C for 12h; stop the reaction, filter, and take The filter cake is dried under a vacuum environment, and the product obtained is myricetin-caffeine eutectic.
Embodiment 3
[0059] Accurately weigh 633.57mg myricetin and 228.76mg caffeine into a 20mL transparent glass bottle, add 10mL methanol, seal the glass bottle and place it in a constant temperature water bath shaker, shake and react at 25°C for 12h; stop the reaction, filter, and take The filter cake is dried under a vacuum environment, and the product obtained is myricetin-caffeine eutectic.
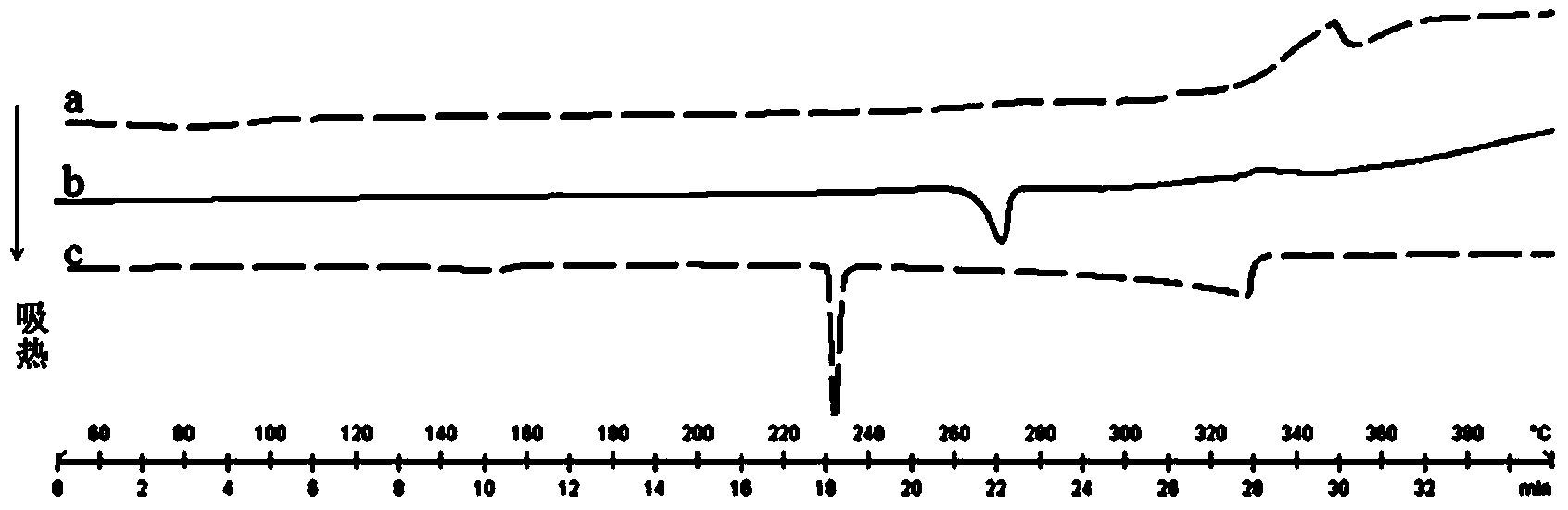
[0060] The differential thermal (DSC) spectrogram of the myricetin-caffeine eutectic that embodiment 1~3 gains is as follows figure 1 Shown: The melting point of the eutectic is different from that of the API and the precursor, and there is an endothermic peak at 275°C, which proves that a new phase is formed.
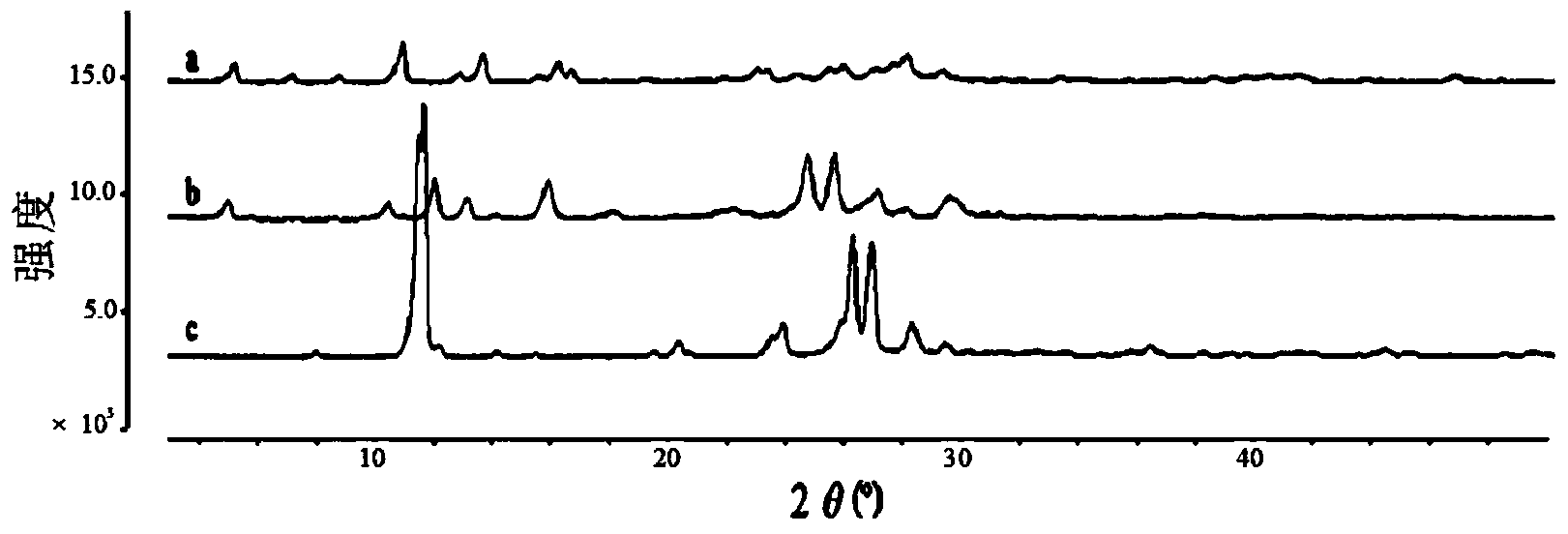
[0061] The X-ray powder diffraction (PXRD) spectrogram of the myricetin-caffeine cocrystal that embodiment 1~3 gains is as follows figure 2 Shown: 25.3°, 10.7°, 12.3°, 13.4°, 14.3°, 16.1°, 17.9°, 18.3°, 21.9°, 22.3°, 24.8°, 25.7°, 27.1°, 28.1°, 29.6° at 2θ A series of characteristic peaks a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com