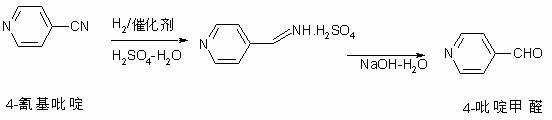Preparation method of 4-pyridylaldehyde
A pyridine carboxaldehyde, cyanopyridine technology, applied in the direction of organic chemistry and the like, can solve the problems of unsuitability for industrial production, a large amount of manganese dioxide waste residue, large environmental pollution, etc., and achieves low cost, short reaction period and good catalytic selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Preparation of Cu-Ni catalyst
[0028] Add 900L of purified water to a 1000L reactor, stir, add 9kg of copper sulfate pentahydrate, then add 30kg of Raney-Ni catalyst (water content 50%), stir for 30 minutes, let stand, take out the supernatant; add 600L of purified water and stir for 30 Minutes, let it stand, and take out the supernatant; repeat the washing 4 to 6 times until the washing water is checked with a barium chloride test solution without precipitation; put the washed catalyst in a bucket, add purified water and soak it for 25 days to obtain Cu- Ni catalyst.
[0029] 2, the preparation method of 4-pyridinecarbaldehyde, comprises the following steps:
[0030] (1) Add 300kg of purified water to a 1000L dissolution reactor, stir, and slowly add 130kg of concentrated sulfuric acid (with a mass fraction above 97%) under brine cooling, add 50.0kg of 4-cyanopyridine below 30°C, and continue stirring for 30 ~60 minutes for complete dissolution of 4-cyanopyridine...
Embodiment 2
[0040] Other parameters of the preparation method of the catalyst are the same as in Example 1, and the soaking time is 35 days.
[0041] The preparation method of 4-pyridinecarbaldehyde comprises the following steps:
[0042] (1) Add 300kg of purified water to a 1000L dissolution reactor, stir, and slowly add 130kg of concentrated sulfuric acid (with a mass fraction above 97%) under brine cooling, add 50.0kg of 4-cyanopyridine below 30°C, and continue stirring for 30 ~60 minutes for complete dissolution of 4-cyanopyridine.
[0043] The above-mentioned 4-cyanopyridine sulfuric acid solution was transferred to a 1000L hydrogenation reactor, stirred, and 15kg (water content 50%) Cu-Ni catalyst plus 80kg purified water was transferred to the hydrogenation reactor. With 0.7MPa N 2 Leak test, then use 0.5MPa H 2 replace N 2 three times. Charge H 2 to 0.5MPa, raise the temperature to 70°C, hydrogenate at 0.6MPa until the reaction system absorbs hydrogen at 0.01-0.03MPa for 20 ...
Embodiment 3
[0050] The preparation method of catalyst is the same as embodiment 1.
[0051] The preparation method of 4-pyridinecarbaldehyde comprises the following steps:
[0052] (1) Add 300kg of purified water to a 1000L dissolution reactor, stir, and under brine cooling, slowly add 235kg of concentrated sulfuric acid (more than 97% mass fraction), add 50.0kg of 4-cyanopyridine below 30°C, and continue stirring for 30 ~60 minutes for complete dissolution of 4-cyanopyridine.
[0053] The above-mentioned 4-cyanopyridine sulfuric acid solution was transferred to a 1000L hydrogenation reactor, stirred, and 15kg (water content 50%) Cu-Ni catalyst plus 80kg purified water was transferred to the hydrogenation reactor. With 0.7MPa N 2 Leak test, then use 0.5MPa H 2 replace N 2 three times. Charge H 2 to 0.5MPa, raise the temperature to 80°C, hydrogenate at 0.8MPa until the reaction system absorbs hydrogen at 0.01-0.03MPa for 20 minutes, then cool down to normal temperature. Row H 2 , w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com