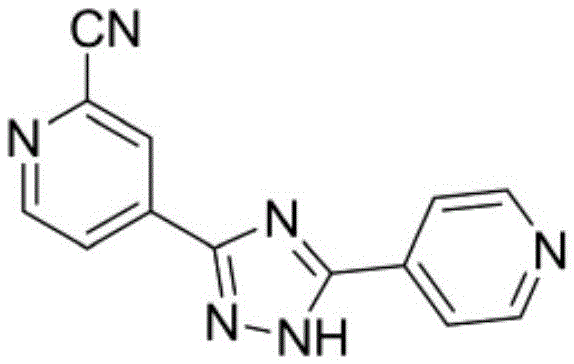
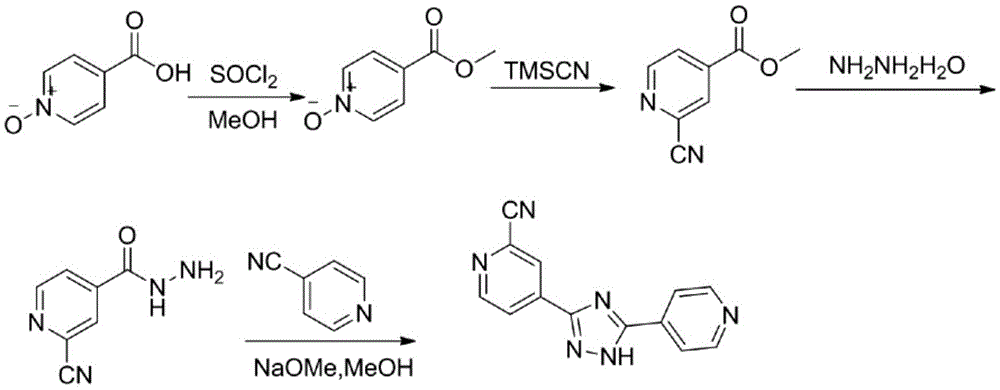
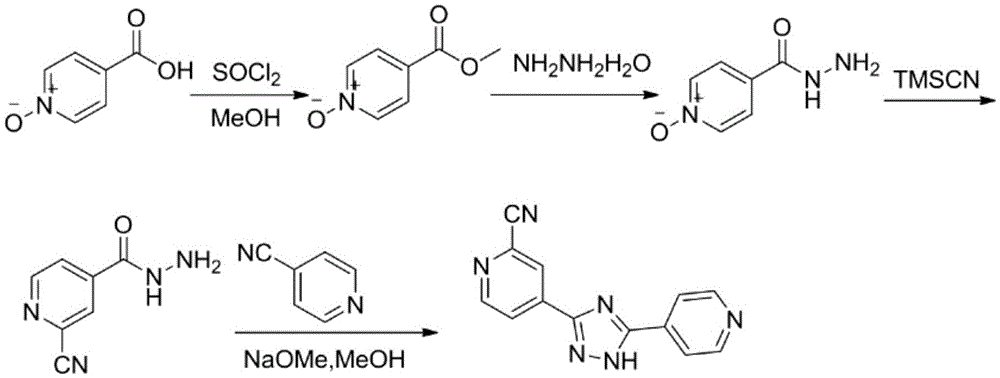
Topiroxostat preparation technology
A preparation process and topicastat technology, applied in the field of medicine and chemical industry, can solve the problems of harsh reaction conditions, high price and high reaction cost, and achieve the effects of improving reaction speed, short synthesis steps and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the synthesis of 2-cyanoisonicotinic acid
[0049] Add 31.5g (0.2mol) of 2-chloroisonicotinic acid, potassium ferrocyanide trihydrate (16.9g, 40mmol), silver iodide (1.4g, 6mmol), polyethylene glycol 400 (3.2g, 8mmol) into the reaction flask ), potassium iodide (1.0g, 6mmol) were dissolved in N, N-dimethylformamide 500mL, reflux and stirred for 2 hours, the reaction solution was filtered, the filtrate was distilled under reduced pressure, the residue was added to ethyl acetate 150mL, and the insoluble matter was removed by filtration. Add dilute hydrochloric acid to the filtrate to adjust the pH to 6.5; wash twice with 50 mL of water, dry the organic phase with anhydrous magnesium sulfate, filter, and evaporate the solvent from the filtrate to obtain crude 2-cyanoisonicotinic acid, add ethanol to the residue and heat After dissolving, cool to 20-25°C for crystallization for 2 hours, filter and dry to obtain 2-cyanoisonicotinic acid (25.2 g) in the form of ...
Embodiment 2
[0050] Embodiment 2: the synthesis of 2-cyanoisonicotinic acid
[0051] Add 2-chloroisonicotinic acid (78.8g, 0.5mol), anhydrous potassium ferrocyanide (36.8g, 100mmol), silver iodide (5.9g, 25mmol), polyethylene glycol 1000 (30.0g, 30mmol), potassium iodide (4.2g, 25mmol) were dissolved in 1000mL of tetrahydrofuran, reflux and stirred for 12 hours, the reaction solution was filtered, the filtrate was distilled under reduced pressure, the residue was added to ethyl acetate 250mL, the insoluble matter was removed by filtration, and the pH of the filtrate was adjusted to 6.2; Wash twice with 50 mL of water, dry the organic phase with anhydrous magnesium sulfate, filter, and evaporate the solvent from the filtrate to obtain crude 2-cyanoisonicotinic acid, add ethanol to the residue, heat to dissolve, and cool to 20 Crystallize at -25°C for 2 hours, filter, and dry to obtain white needle-like crystals of 2-cyanoisonicotinic acid (66.0 g). The HPLC purity is 98.6%, and the yield is...
Embodiment 3
[0052] Example 3: Synthesis of 2-cyanoisonicotinic acid
[0053] Add 2-chloroisonicotinic acid (78.8g, 0.5mol), potassium ferrocyanide trihydrate (63.4g, 150mmol), silver iodide (8.2g, 35mmol), polyethylene glycol 1000 (50g, 50mmol) into the reaction flask ), potassium iodide (8.3g, 50mmol) were dissolved in N-methylpyrrolidone 1000mL, 100 ° C stirring reaction for 5 hours, the reaction solution was filtered, the filtrate was distilled under reduced pressure, the residue was added to ethyl acetate 300mL, the insoluble matter was removed by filtration, and the filtrate was added to Adjust the pH to 6.6 with dilute hydrochloric acid; wash twice with 100 mL of water, dry the organic phase with anhydrous magnesium sulfate, filter, and evaporate the filtrate to obtain crude 2-cyanoisonicotinic acid, add ethanol to the residue and heat to dissolve Afterwards, cool to 20-25° C. for crystallization for 2 hours, filter, and dry to obtain 2-cyanoisonicotinic acid (63.0 g) in the form of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com