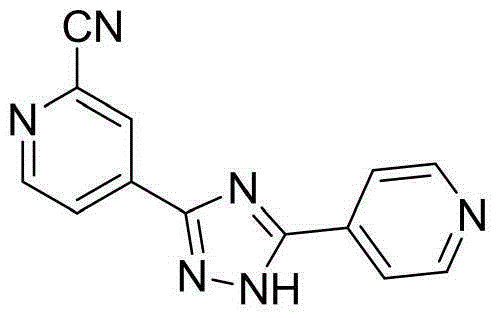
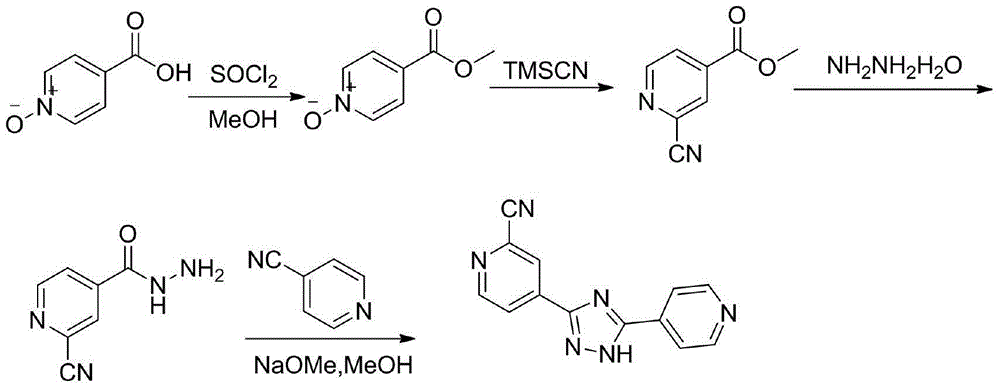
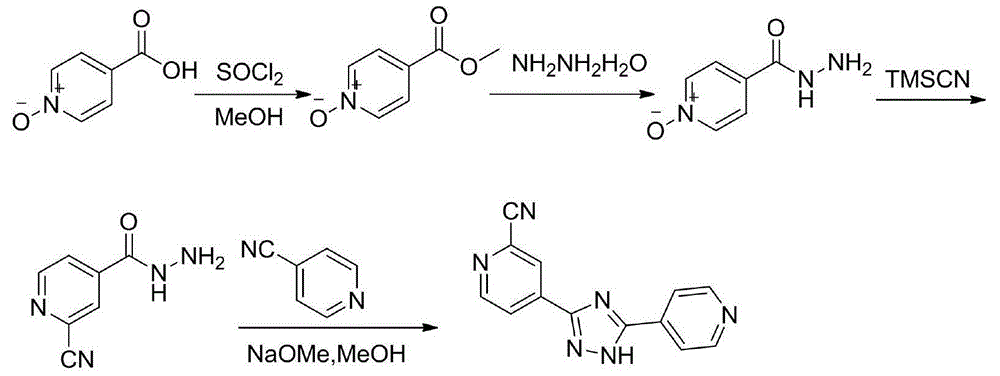
Preparation technology for 5-(2-cyano4-pyridyl)-3-(4-pyridyl)-1,2,4-troazole
A preparation process, pyridyl-based technology, applied in the field of medicine and chemical industry, can solve the problems of only 30% yield, high post-processing requirements, cumbersome post-processing process, etc., to achieve simplified production operations, low toxicity and environmental pressure, The effect of shortening the process time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation method of compound III
[0038] Method 1: Add compound II (137.0g, 1.0mol) to a mixture of phosphorus oxychloride (137g, 1.0mol) and N,N-dimethylformamide (292g, 4.0mol) cooled at 0-5°C In the solution, keep stirring at 0-5°C for 6 hours. After the reaction, the I 2 (279.4g, 1.1mol) and 28% ammonia water (674ml, 10mol) were added to the reaction liquid, and the stirring reaction was continued for 3 hours at 20-25°C. Pour the reaction solution into a saturated aqueous solution of sodium sulfite, extract with dichloromethane, separate the organic phase and dry it with anhydrous sodium sulfate, filter and evaporate to dryness, add ethanol to the residue and heat to dissolve, then cool to 20-25°C to crystallize for 2 hours , filtered and dried to obtain white needle crystals, 66.7g, yield: 41.2%
[0039] Method 2: Compound II (137.0g, 1.0mol) was added to phosphorus oxychloride (150.7g, 1.1mol) and N,N-dimethylformamide (292.0g, 4.0mol) cool...
Embodiment 2
[0041] Embodiment 2: the preparation method of compound IV
[0042] Method 1: add compound III (64.8g, 0.4mol) and methanol (650ml) respectively, after stirring evenly, then add 80% hydrazine hydrate (37.5g, 0.6mol), after adding, the reaction system shows yellow suspension, 20 The reaction was stirred at -25°C for 2 hours, followed by TLC. After the reaction was completed, it was filtered, washed with 30ml of ice methanol, and dried to obtain 31.2g of a light yellow solid. Yield: 48.1%
[0043] Method 2: add compound III (64.8g, 0.4mol) and methanol (650ml) respectively, after stirring evenly, then add 80% hydrazine hydrate (37.5g, 0.6mol), after the addition, the reaction system shows a yellow suspension, 0 Stir the reaction at ℃ for 4 hours, follow the reaction process by TLC, after the reaction is completed, filter, wash with 20ml ice methanol, and dry to obtain 26.2g, light yellow solid, yield: 40.5%
[0044] Method 3: add compound III (64.8g, 0.4mol) and ethanol (650ml)...
Embodiment 3
[0047] Embodiment 3: the preparation method of crude product
[0048] Method 1: Add the raw material 4-cyanopyridine (20.8g, 0.2mol), dissolve it in 350ml of methanol, add 27% methanol solution of sodium methoxide (40g, 0.2mol) at 20-25°C, and react the solution at 20-25 °C and stirred for 2 hours, during which the reaction solution was a clear and transparent solution. Compound IV (32.4g, 0.2mol) was added to the reaction solution, followed by heating to reflux, 2-cyanoisonicotinic acid hydrazide gradually dissolved, heating to reflux for 14 hours, a large amount of solids were precipitated, the reaction was completed, and cooled to 20-25 °C, the solid was filtered, the filter cake was washed twice with 150 ml of methanol, and dried to obtain 30.0 g of a yellow solid, with a yield of 60.5%.
[0049] Method 2: Add the raw material 4-cyanopyridine (20.8g, 0.2mol), dissolve it in 350ml of ethanol, add 27% methanol solution of sodium methoxide (40g, 0.4mol) at 20-25°C, and the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com