While the exact
etiology of the syndrome that causes
heart failure is not fully understood, the primary cause of CHF is left ventricular dysfunction (i.e., the inability of the heart to properly and adequately fill or empty blood from the left
ventricle with adequate efficiency to meet the metabolic needs of the body).
With existing pharmacological, surgical and device-based therapies symptoms can be alleviated, but the quality of a patient's life remains significantly impaired.
Further morbidity and mortality associated with the
disease is exceptionally high.
Decreases in systolic contraction can lead to
cardiomyopathy, which further exacerbates the localized,
ischemia damaged tissue or AMI insult into a global impairment, thereby leading to episodes of arrhythmia, progressive
pump failure and death.
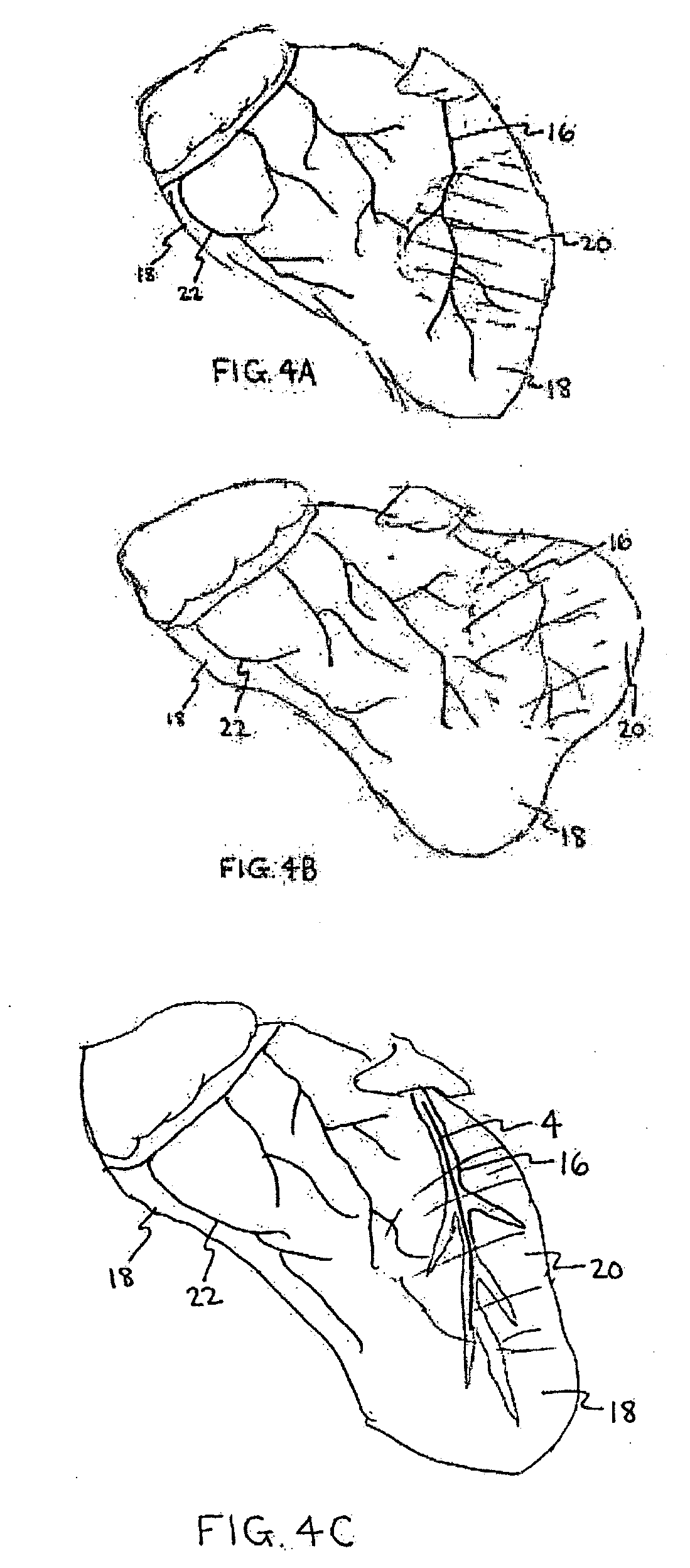
Ischemia-damaged and / or infarct damaged heart
muscle tissue results in progressive
softening or degeneration of cardiac tissue.
These ischemic and infarcted zones of the heart muscle wall have limited, if not complete loss of tissue contractile functionality and overall
physical integrity and present an analogous situation to those presented by vascular aneurysms.
With this enlargement, the heart's burden is increased to pump more blood with each pump cycle.
With this bulging, the heart's natural contraction mechanism is dissipated and attenuated, resulting in a marked and progressing decrease in
cardiac output.
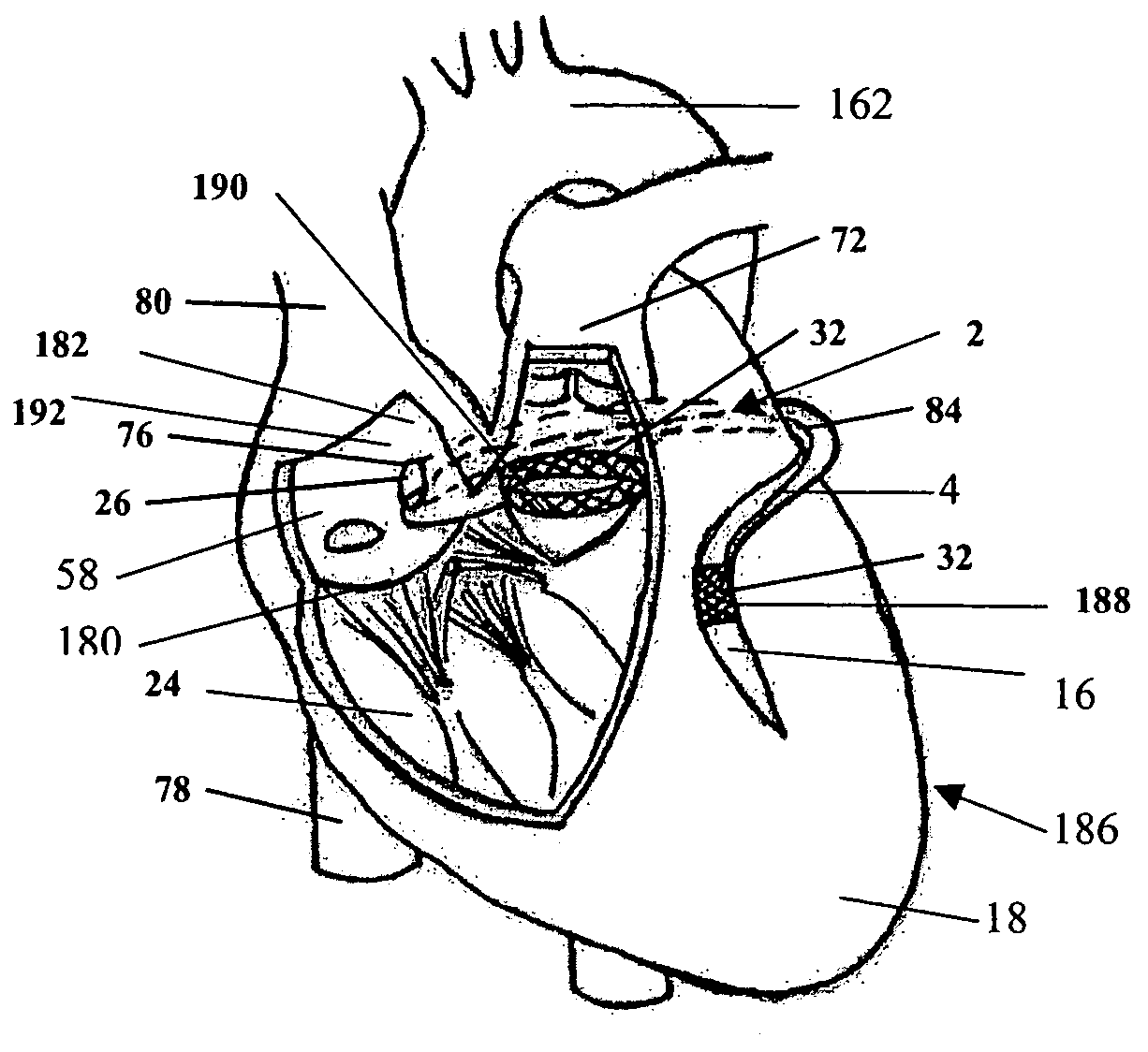
Any damage or impairment in function of any of these key components can render the valve structure incompetent.
Impairment of valve function, due to independent factors (i.e., a concomitant valve
pathology) or dependent factors (i.e., valve dilation related to
dilated cardiomyopathy), can result in valvular insufficiency further exacerbating the degenerative CHF cycle.
However, there still remains no definitive cure for CHF.
The procedure is known to provide some symptomatic improvement, but is controversial with regard to its ability to enable active improvement of cardiac performance.
In
spite of the positive outcome on relieving some of the symptoms, the procedure is highly invasive, requiring access to the heart via a stemotomy, expensive, complex and of unknown durability (due to the muscle wrap
blood flow requirements and
fibrosis issues).
While innovative, the procedure is highly invasive, traumatic and costly.
Further, the actual
volume reduction results in a reduction in valve competence and elicits the associated regurgitation.
While being generally successful and routine in surgical practice today, these procedures are also costly, highly invasive and are still have significant associated morbidity and mortality.
Still, the use of such devices is limited by high costs and a lack of substantial,
clinical evidence warranting their use.
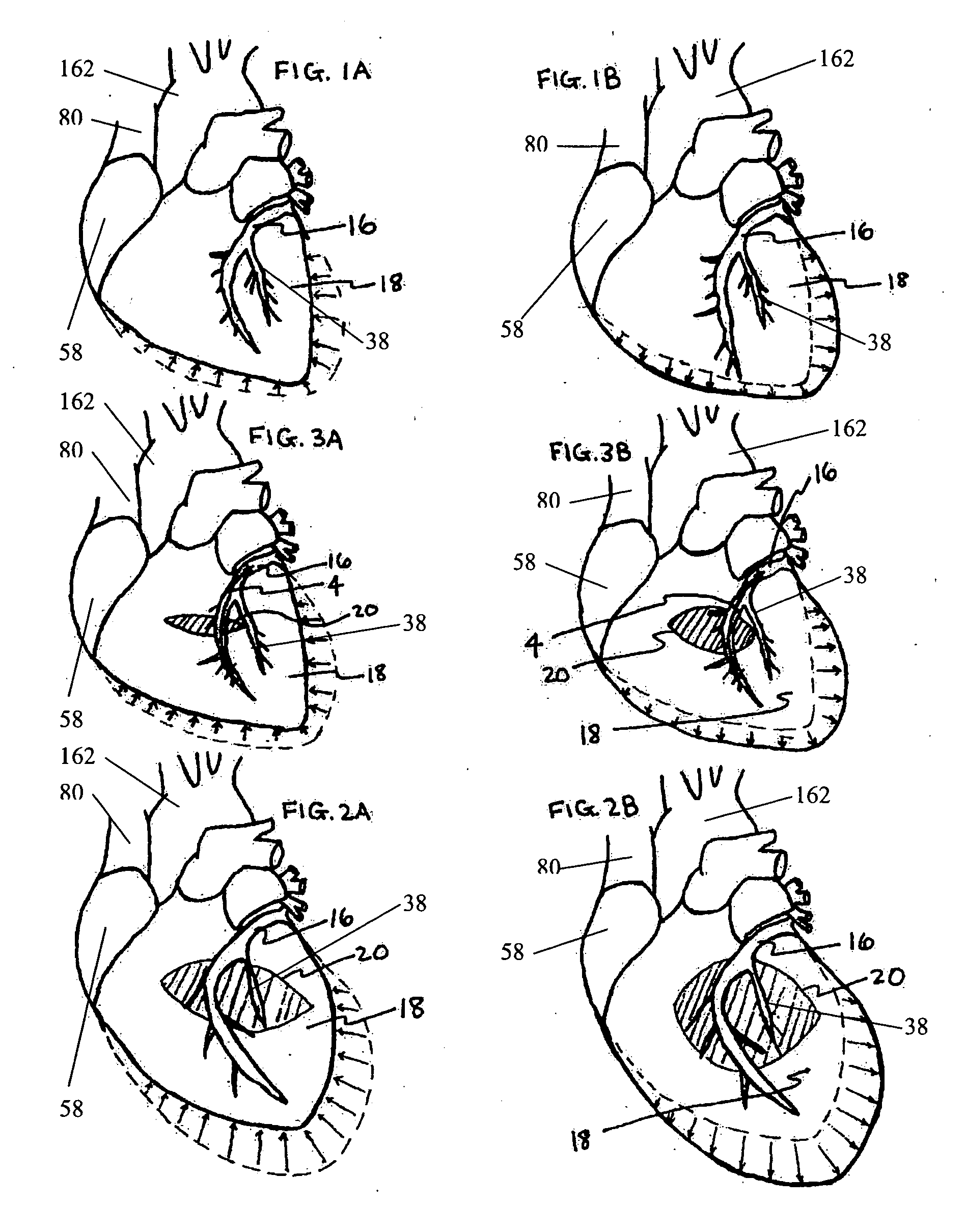
As a result, these types of approaches require unnecessary positioning of the devices over healthy (
non local, undamaged) areas or zones of the heart affecting the entire organ when the
primary treatment is usually focused is on the left
ventricle or the
mitral valve annulus.
Such non-localized treatment can elicit iatrogenic conditions such as undesired valvular dysfunction and / or constrictive
physiology due to over restriction of the heart by such restraints.
Transplants represent a massive challenge with donor hearts generally in short supply and with the transplant
surgery itself presenting a high risk, traumatic and costly procedure.
In
spite of this, transplants present a valuable, albeit limited, upside, increasing
life expectancy of end stage congestive heart failure patient from less than one year up to a potential five years.
In view of the above, it should be evident that there is currently no ideal treatment among the various surgical, pharmacological, and device-based approaches to treat the multiple cardiac and non-cardiac factors implicated with the syndrome of CHF.
 Login to View More
Login to View More  Login to View More
Login to View More