Method for determining content of 4-cyanopyridine and impurities of 4-cyanopyridine in isoniazide starting material
A kind of technology of cyanopyridine, determination method, applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
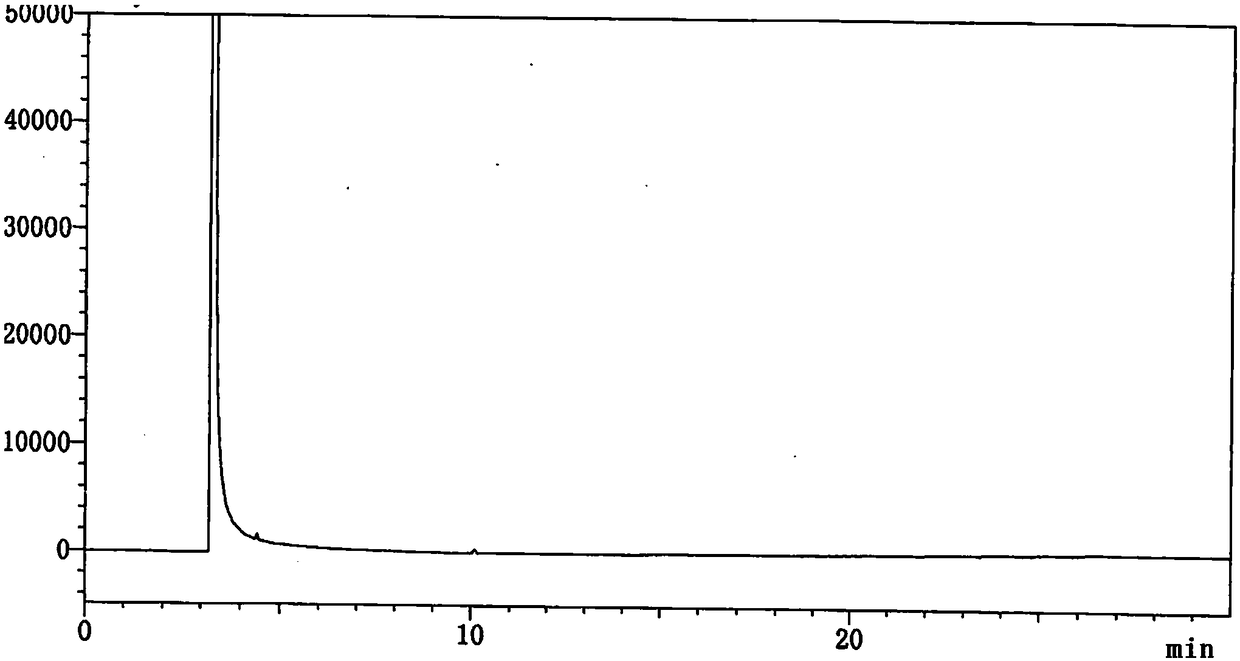
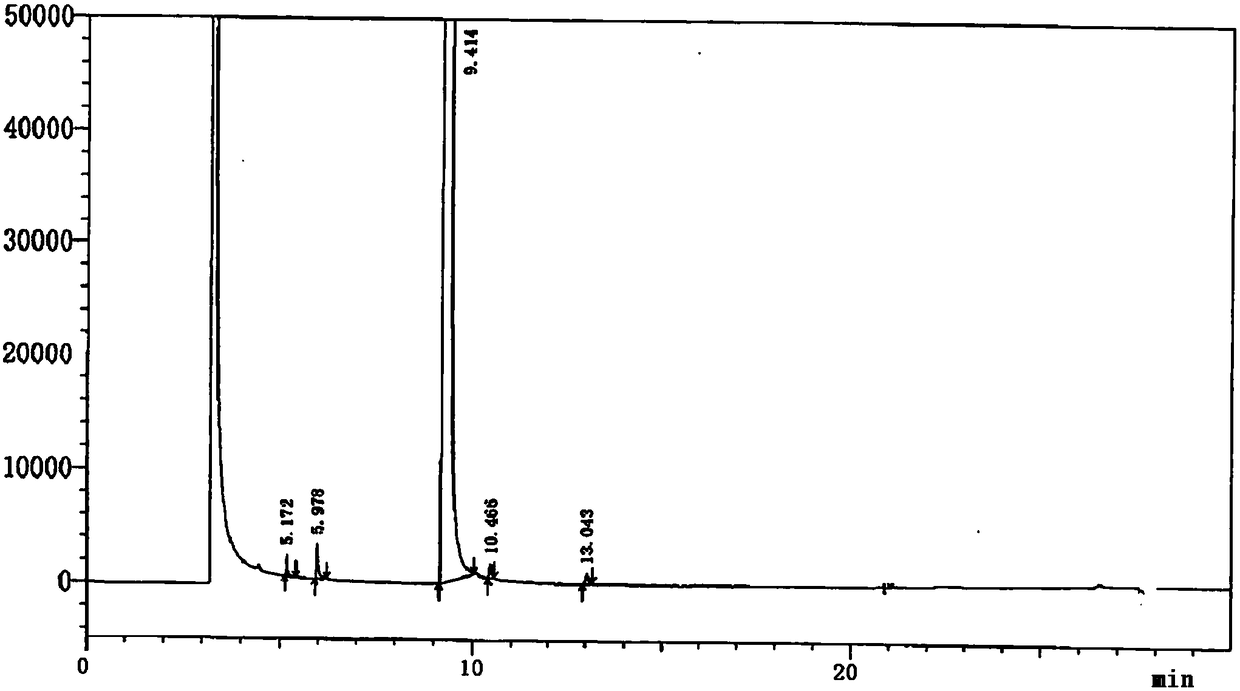
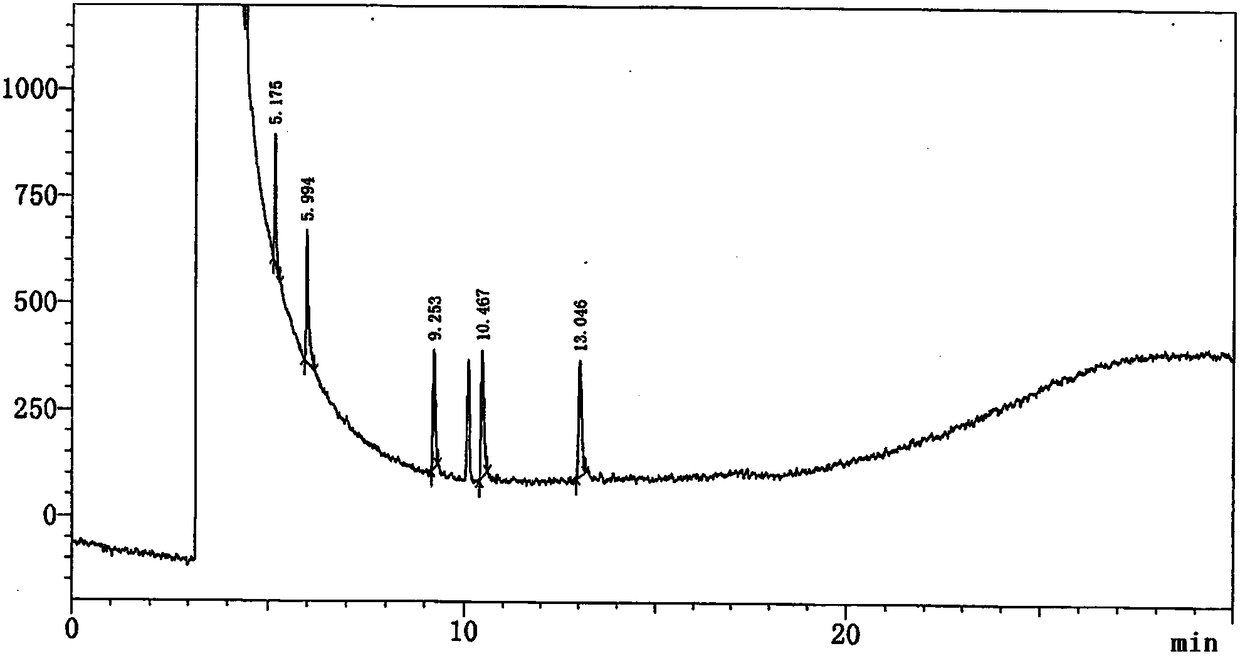
[0099] Embodiment 1 Determination of related substances of 4-cyanopyridine SM1
[0100] 1. Instruments and conditions
[0101] Table 1 Instrument and chromatographic conditions
[0102]
[0103]
[0104] 2. Experimental steps
[0105] (1) Take an appropriate amount of impurity SM1a, impurity SM1c, impurity SM1d, impurity SM1e and this product, add methanol to dissolve and dilute to make about 0.01mg of impurity SM1a, impurity SM1c, impurity SM1d, impurity SM1e and SM1 10mg per 1ml solution, as a system suitability solution.
[0106] (2) Take this product, weigh it accurately, add methanol to dissolve and dilute to make a solution containing about 10 mg per 1 ml, as the test solution.
[0107] (3) Accurately measure 1ml of the test solution, put it in a 100ml measuring bottle, dilute to the mark with methanol, shake well, and use it as a control solution.
[0108] (4) Weigh an appropriate amount of each impurity reference substance and the test sample, add diluent met...
Embodiment 2
[0121] Embodiment 2 Several batches of 4-cyanopyridine related substance assay results
[0122] According to the method of Example 1, several batches of 4-cyanopyridine samples from our company were tested for related substances. The calculation results are shown in Table 3.
[0123] Table 3 Test results of several batches of samples
[0124]
[0125] The method provided by the invention only uses gas chromatography to separate and measure 4-cyanopyridine and its related substances, and can effectively separate other solvent peaks so that it does not interfere with the detection of related substances. The method has the advantages of good separation, strong specificity, high sensitivity, and simple operation, and has the advantages of simplicity and speed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com