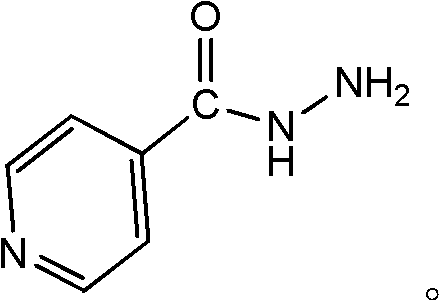
Method for controlling impurities of isoniazid
A technology of isoniazid and impurities, applied in the field of control of isoniazid impurities, to achieve the effect of easy control and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 isoniazid
[0037] Add 120g MnO to the 2000ml three-necked bottle 2 , then hot-melted 600g (5.76mol) 4-cyanopyridine (HPLC chromatographic purity is 99.8%, wherein the content of impurity INH-C is 0.07%, the content of impurity INH-D is 0.06%, the content of impurity INH-B The content is 0.07%) and 360g of water are added, the temperature is raised to 95 ℃ for 3 hours, and after 3 hours, 600g of water is added and filtered. The filtrate was concentrated under reduced pressure, and when about 720g of water was reclaimed, the concentration was stopped (in the concentrate that was recorded by HPLC, the content of INH-A was 0.30%, and the content of INH-F was 1.20%), nitrogen protection, 894g (17.88 mol) hydrazine hydrate was added, the temperature was raised, and the time was started at 90°C. After 2.5 hours of heat preservation at 90°C, the diluent (450g of water) was added, the temperature was raised appropriately, and dissolved to obtain...
Embodiment 2
[0038] The refining of embodiment 2 isoniazid
[0039] Add the aqueous solution of the crude isoniazid product prepared in Example 1 to a 2000ml three-necked flask, add 5g of activated carbon, heat to 75°C, keep warm for half an hour, filter, and the filtrate is naturally cooled for 4 hours, then cooled to 0°C, filtered, The refined product is obtained once, and the refined product is recrystallized once by the same method, and dried to obtain the finished product of isoniazid.
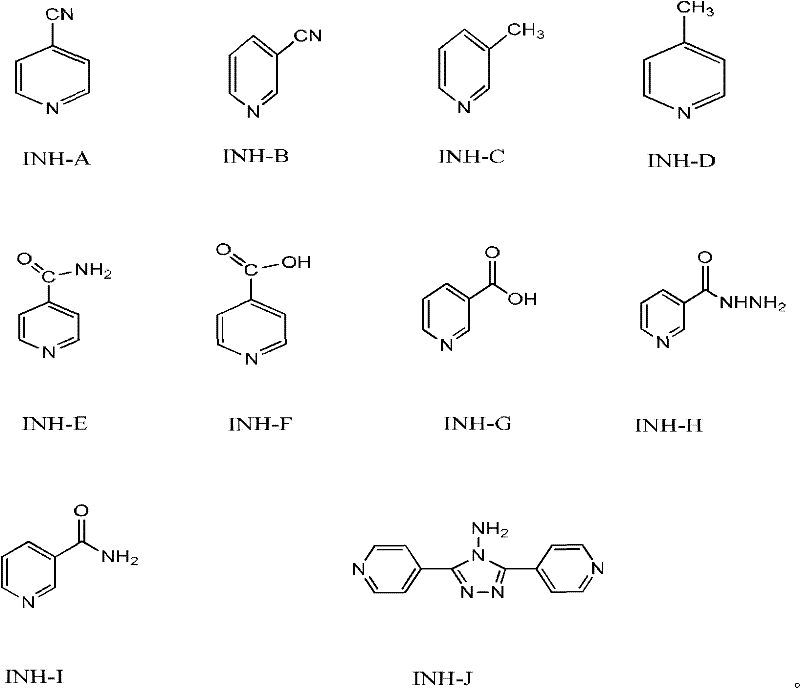
[0040] Adopt the purity of the isoniazid finished product of HPLC method test gained, the content of relevant impurity, the result is as follows:
[0041] The purity of the finished product of isoniazid is 99.91%; the impurity INH-A is not detected, the impurity INH-B is not detected, the impurity INH-C is not detected, the impurity INH-D is not detected, and the content of the impurity INH-E is 0.02 %, the content of INH-F is 0.01%, the content of INH-G is 0.02%, the impurity INH-H is not detected, ...
Embodiment 3
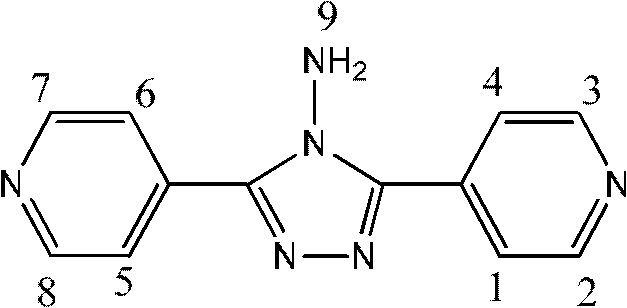
[0042] Example 3 Preparation of INH-J
[0043] Add 13g (0.1mol) of isonicotinic acid into a 250ml flask, weigh 80g (1.6mol) of hydrazine hydrate, and gradually raise the temperature to 140°C for 24 hours. Finally a yellow oily liquid was obtained. After the product was cooled and solidified, it was recrystallized with acetone to obtain 4 g of the product.
[0044] Confirmation of product structure:
[0045]
[0046] NMR data:
[0047] Hydrogen spectrum ( 1 H-NMR) and attribution
[0048] Numbering
δ, ppm
1,4,5,6
8.075-8.086
4
2,3,7,8
8.803
4
9
6.540
2
[0049] Infrared spectral data:
[0050]3299.10cm -1 : v N-H
[0051] 3173.13em -1 : v N-H
[0052] 1600.53cm -1 : v C = N
[0053] Mass spectrometry data: the quasi-molecular ion peak in the spectrogram is [M-H] + The mass number of the sample is m / z237, and the relative molecular mass of the sample is 238, which is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com