Solid Phase Fragment Synthesis of Thymosin α1
A synthetic method and technology of thymosin, which is applied in the field of solid-phase fragment synthesis of thymosin α1, can solve the problems of complex preparation process of pseudoproline dipeptide, difficulty in large-scale preparation, and high price, and achieve low cost and reduced production The effect of shortening the cost and production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] According to the "fragment Ac(1-4) + stepwise condensation of 5-14 amino acids + Fmoc (15-22) + (23-28)-Wang Resin" synthesis strategy to synthesize the three required fragments, the process flow chart is as follows:
[0048] Ⅰ. Preparation (23-28) - Wang Resin
[0049]
[0050] Ⅱ. Preparation of Fragment Fmoc(15~22) Fully Protected Crude Peptide
[0051]
[0052] Ⅲ. Condensation of fragment Fmoc (15~22) fully protected crude peptide and (23~28)-Wang Resin, and gradually condense to the fifth amino acid
[0053]
[0054] Ⅳ. Preparation of Fragment Ac(1~4) Fully Protected Crude Peptide
[0055]
[0056] Ⅴ. Fragment Ac(1~4) fully protected crude peptide is condensed with (5~28)-Wang Resin, and cracked into crude peptide
[0057]
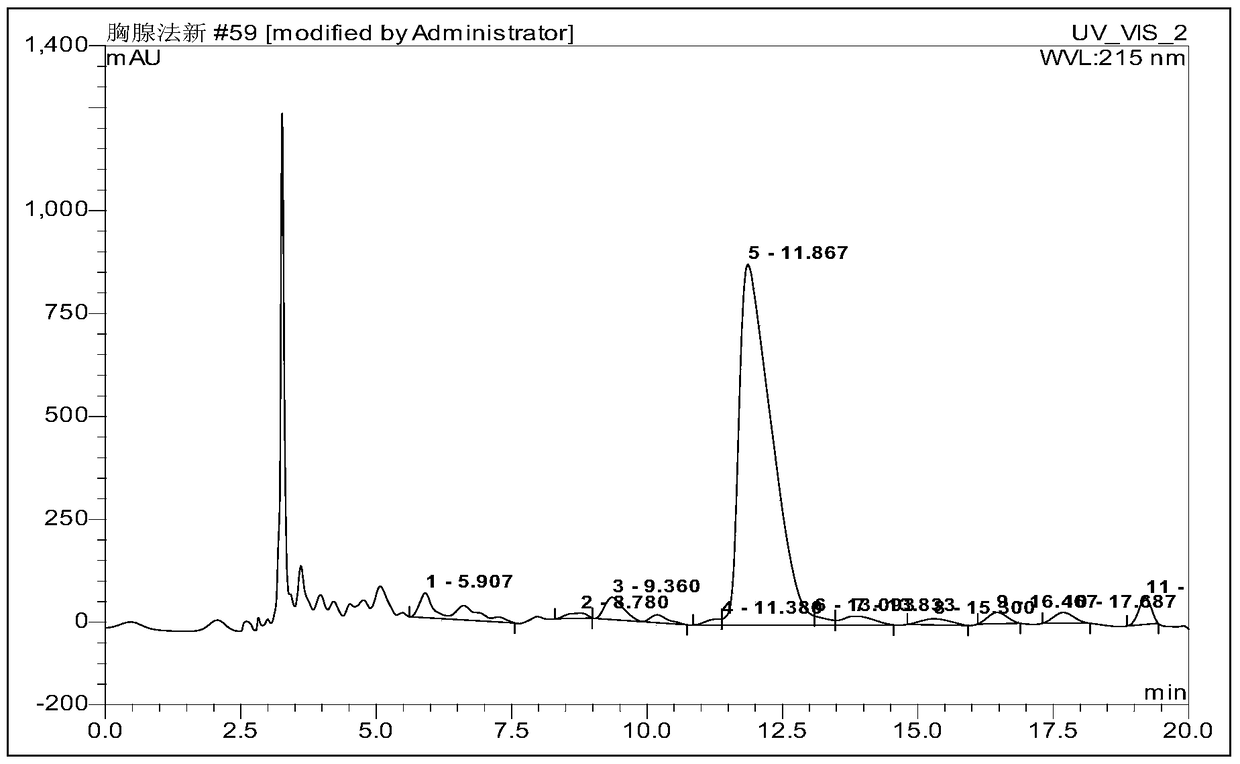
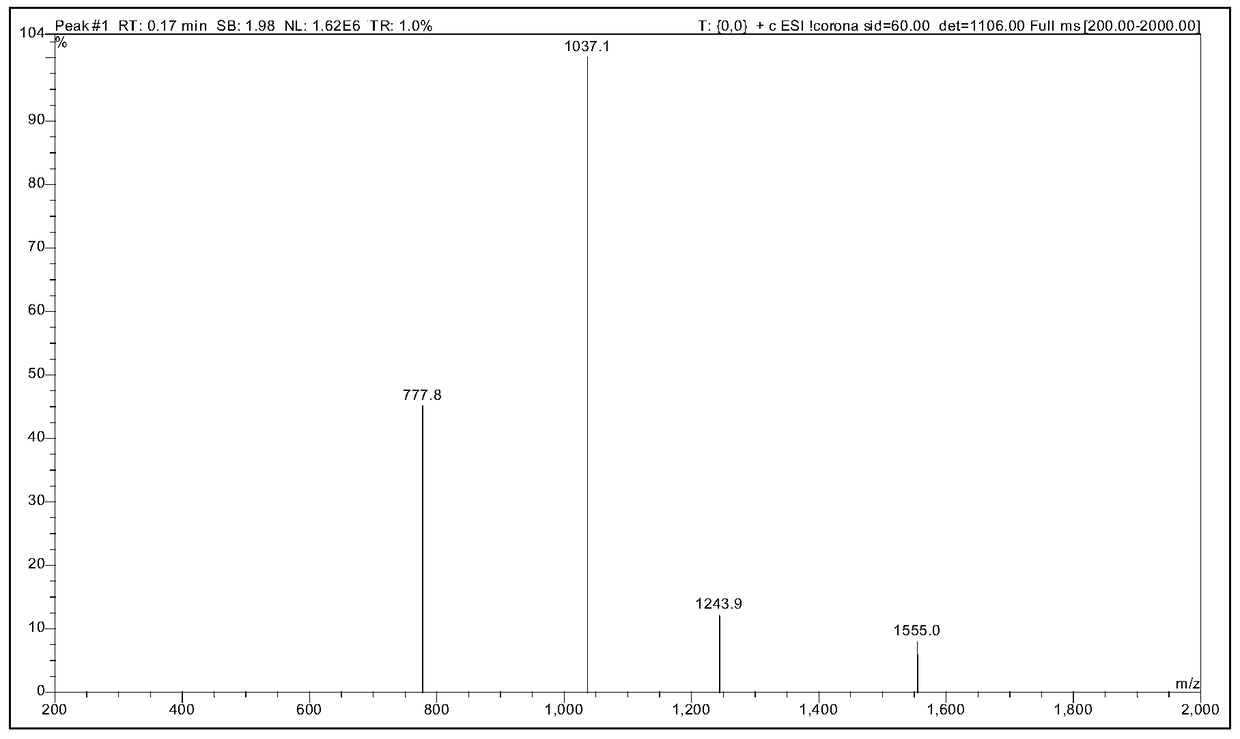
[0058] Ⅵ. Purification and desalting of crude peptide
[0059]
[0060] (1) Synthesis of fully protected peptide resin of C-terminal fragment A (AA23-AA28) H-Val-Glu(OtBu)-Glu(OtBu)-Ala-Glu(OtBu)-Asn(Trt)-Wang Resin
[0061...
Embodiment 2
[0096] (1) Four fragments were synthesized according to the synthesis strategy of "fragment Ac(1~2)+ stepwise condensation of amino acids at positions 3~14+Fmoc(15~24)+(25~28)-WangResin". The process flow chart is as follows:
[0097] Ⅰ. Preparation (25~28)-Wang Resin
[0098]
[0099] Ⅱ. Preparation of Fmoc(15~24) Fully Protected Crude Peptide
[0100]
[0101] Ⅲ. Condensation of fragment Fmoc (15~24) fully protected crude peptide and (25~28)-Wang Resin, and gradually condense to the third amino acid
[0102]
[0103] Ⅳ. Preparation of Fragment Ac(1~2) Fully Protected Crude Peptide
[0104]
[0105] Ⅴ. Condensation of fragment Ac(1~2) fully protected crude peptide with (3~28)-Wang Resin, and cleavage into crude peptide
[0106]
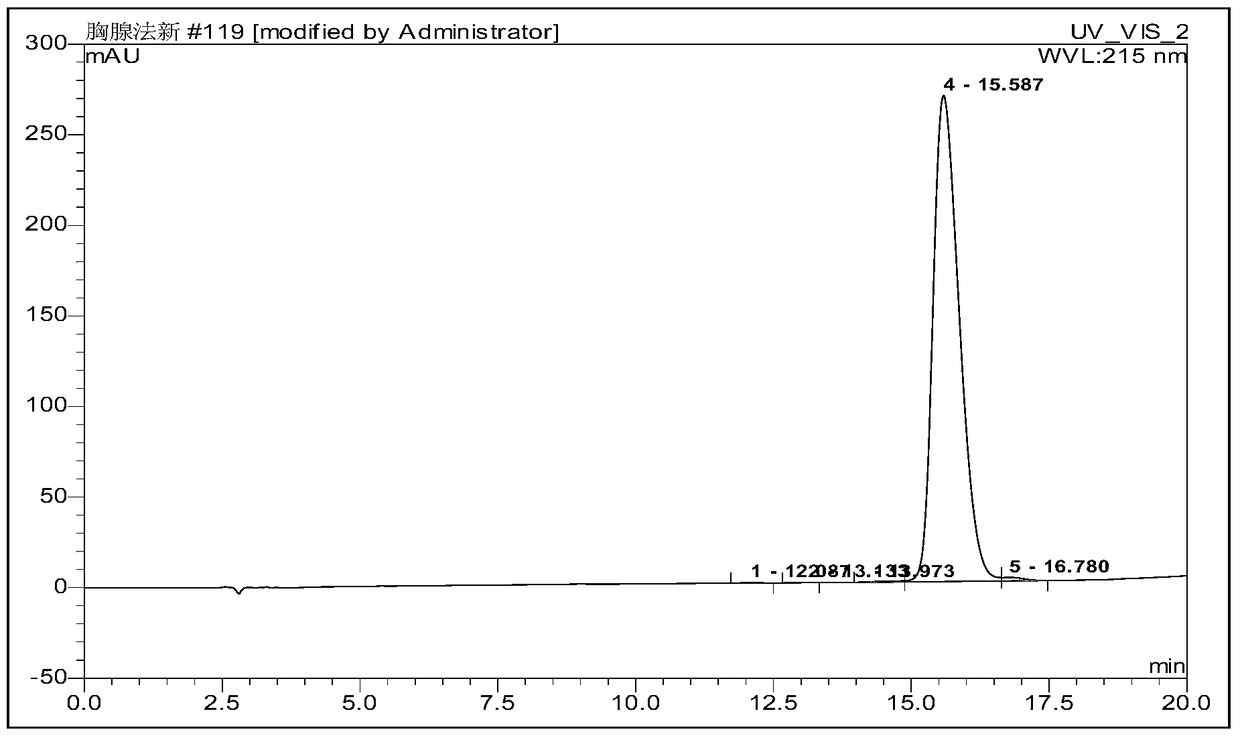
[0107] Ⅵ. Purification and desalting of crude peptide
[0108]
[0109] The specific operation is as follows:
[0110] a. Synthetic Fragment (25~28)-Wang Resin: Weigh 920.0g Wang Resin (1.2mmol / g) into a 10L reactor, add an appr...
Embodiment 3
[0126] (1) Synthesize the three required fragments according to the synthetic strategy of "Ac+ Fmoc(1~4)+ stepwise condensation of amino acids at positions 5~14+Fmoc(15~22)+(23~28)-WangResin". The process flow chart is as follows :
[0127] Ⅰ. Preparation (23~28)-Wang Resin
[0128]
[0129] Ⅱ. Preparation of Fragment Fmoc(15~22) Fully Protected Crude Peptide
[0130]
[0131] Ⅲ. Condensation of fragment Fmoc (15~22) fully protected crude peptide and (23~28)-Wang Resin, and gradually condense to the fifth amino acid
[0132]
[0133] Ⅳ. Preparation of Fragment Fmoc(1~4) Fully Protected Crude Peptide
[0134]
[0135] Ⅴ. Fragment Fmoc(1~4) fully protected crude peptide is condensed with (5~28)-Wang Resin, acetylated and cracked into crude peptide
[0136]
[0137] Ⅵ. Purification and desalting of crude peptide
[0138]
[0139] The specific operation is as follows:
[0140] a. Synthesis of (23~28)-Wang Resin: Weigh 1100.0g Wang Resin (1.0mmol / g) into a 10L ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com