Preparation method for 4-(3-fluorine phenyl)-2,2-phenyl-5-(4-(methylmercapto-)phenyl) furan-3(2H)-ketone
A technology of fluorophenyl and methylthio, which is applied in the field of organic synthesis, can solve the problems of low product purity, complicated production methods, and high production costs, and achieve the effects of simple process, reduced production costs, and improved production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
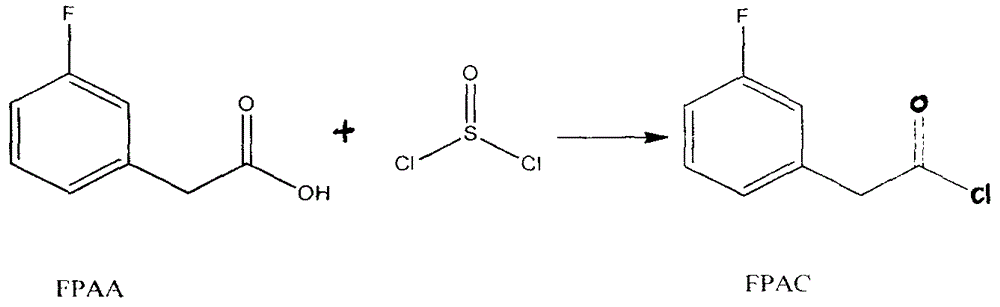
[0022] 1) Preparation of m-fluorophenylacetyl chloride (FPAC)
[0023] Add 305g m-fluorophenylacetic acid successively in a 1L three-necked flask. 500ml of thionyl chloride and 2ml of DMF, stirred and heated to reflux, the temperature was 110°C, the time was 4h, the reaction was completed, and the thionyl chloride was removed by atmospheric distillation, and the product at 64°C / 5mmHg and 320g wine red product were collected. The rate is 82.3%.
[0024] 2) Synthesis of 3-bromo-3-methyl-2-oxobutylcyanide (BBC)
[0025] 557g of BBB (2-bromo-2-methylpropionyl bromide) and 283g of trimethylsilyl cyanide were added to a 1L three-necked flask, the temperature was controlled at 85°C and the reaction was stirred for 3 hours, then cooled to room temperature and collected at 70°C / The product of 79mmHg is 384g, and the yield is 90.04%.
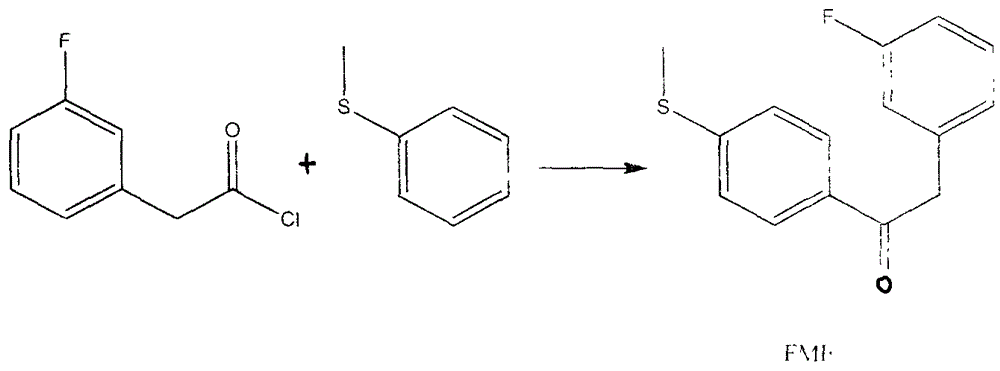
[0026] 3) 2-(3-fluorophenyl)-1-(4-(methylthio)phenyl)ethanone (FME)
[0027] In a 3L three-neck flask, add 2500ml of dichloromethane, quickly add (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com