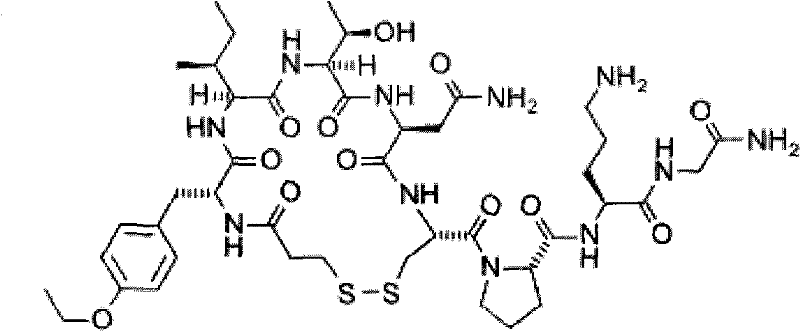
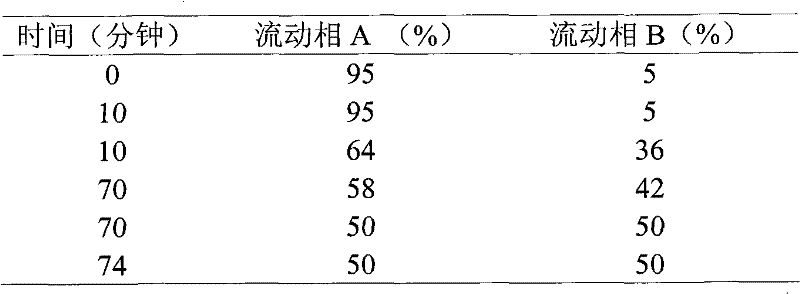
A kind of solid phase synthesis atosiban method
A technology of atosiban and solid-phase synthesis, which is applied in the preparation methods of peptides, chemical instruments and methods, organic chemistry, etc., can solve the problems of increasing the risk of product quality, affecting the production schedule, and production operation restrictions, and reducing the Loss of target product, low production cost, and the effect of reducing waste of solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of Fmoc-Gly-resin
[0055] Add 100.00 g of Rink Amide resin to the reaction column, add 550 ml of DCM / wash for 3 times, then add 550 ml of DMF / wash for 3 times, add 550 ml of prepared 30% piperidine / DMF solution to the glass reaction column, and stir. The reaction was carried out for 30 min, the reaction solution was removed, 550 ml of DMF was added for washing 6 times, and the deprotection effect was detected with 5% ninhydrin ethanol solution. Weigh 23.79g of Fmoc-Gly-OH and 10.80g of HOBT, add N, N-dimethylformamide (DMF) and stir to dissolve, add 12ml of DIPCDI after complete dissolution, stir evenly and add to the glass reaction column, stir and react 24 Hour. After the completion of the reaction, the reaction solution was removed, washed 3 times with 550 ml of DMF, then washed 3 times with 550 ml of DCM, and finally washed 3 times with 550 ml of methanol, drained, poured out, and dried in a vacuum drying box. 12h. It was taken out and weig...
Embodiment 2
[0056] Example 2 Preparation of Fmoc-Orn(Boc)-Gly-resin
[0057] 103.19 g of Fmoc-Gly-resin was added to the reaction column, 550 ml of DCM was added to wash 3 times, and 550 ml of DMF was added to wash 3 times. Add 550ml of prepared 30% piperidine / DMF solution to the glass reaction column, stir for 30min, remove the reaction solution, add 550ml of DMF / wash for 6 times, use 5% ninhydrin ethanol solution to detect the deprotection effect . Weigh 108.34 g of Fmoc-Orn(Boc)-OH and 32.21 g of HOBT, add DMF and stir to dissolve, after complete dissolution, add 37 ml of DMF solution of 1 mmol / ml DIPCDI, and stir evenly. The prepared amino acid coupling solution was added to the reaction column, and the reaction was stirred for 90 min. The reaction solution was removed, washed 6 times with DMF 550ml / time, and the coupling effect was detected with 5% ninhydrin ethanol solution.
Embodiment 3
[0058] Example 3 Preparation of Fmoc-Pro-Orn(Boc)-Gly-resin:
[0059] Add 550ml of prepared 30% piperidine / DMF solution to the glass reaction column, stir for 30min, remove the reaction solution, add 550ml of DMF / wash for 6 times, use 5% ninhydrin ethanol solution to detect the deprotection effect . Weigh 32.21 g of Fmoc-Pro-OH and HOBT, add DMF and stir to dissolve, and after complete dissolution, add 37 ml of a DMF solution of 1 mmol / ml DIPCDI, and stir evenly. The prepared amino acid coupling solution was added to the reaction column, and the reaction was stirred for 90 min. The reaction solution was removed, washed 6 times with DMF550ml / time, and the coupling effect was detected by sampling with 5% ninhydrin ethanol solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com