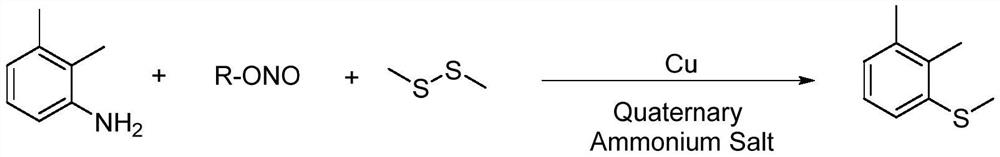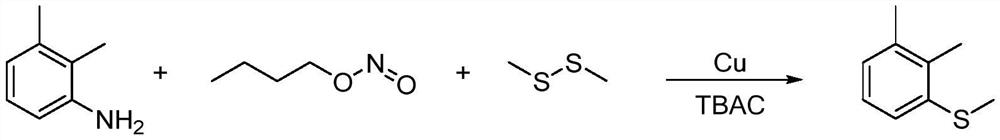Method for preparing 2, 3-dimethyl thioanisole
A technology of dimethyl anisole and dimethyl disulfide, which is applied in the field of fine chemical intermediate synthesis, can solve the problems of cumbersome post-processing, high consumption and high cost, avoid post-processing steps, simplify processes, Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
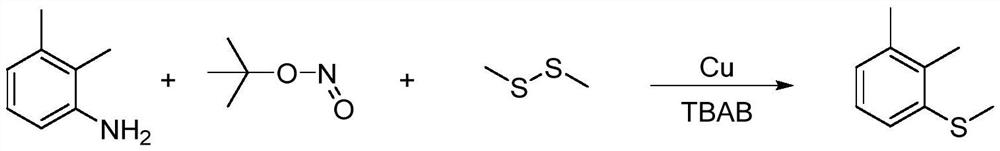
[0037] In a 250mL four-necked flask, add 2.57g (0.04mol, 99.5%) copper powder, 0.5g tetrabutylammonium bromide (TBAB), 56.97g (0.6mol, 99%) dimethyl disulfide , stirred, and the reaction temperature was controlled to be 20°C, and 17.35g (0.16mol, 95%) of tert-butyl nitrite was added dropwise for 0.5 hours. , 99%) 2,3-dimethylaniline, the dropping time is 1.5 hours, after the dropping, the temperature is raised to 50°C, reacted for 4 hours, sampling is carried out in the gas chromatography, and the content of 2,3-dimethylaniline is less than 0.2 %, stop the reaction. The reaction process can be represented by the following reaction equation:
[0038]
[0039] The reaction material was cooled to 25°C, filtered, and the residue was washed twice with dimethyl disulfide, and the amount of dimethyl disulfide was 10 g each time. The organic phases were combined and desolvated under reduced pressure at a vacuum of 4KPa. 15.69 g of crude product was obtained, the quantitative cont...
Embodiment 2
[0041]In a 250mL four-necked bottle, add 6.43g (0.1mol, 99.5%) copper powder, 0.12g tetrabutylammonium chloride (Tetrabutylammonium chloride, TBAC), 23.74g (0.25mol, 99%) dimethyl disulfide , stirred, and the reaction temperature was controlled to be 30°C, and 21.68g (0.2mol, 95%) of n-butyl nitrite was added dropwise for 0.5 hours. After the addition, the temperature was raised to 60°C, 12.22g (0.10mol , 99%) 2,3-dimethylaniline, the dropping time is 0.5 hours, after the drop is completed, the temperature is raised to 75 ° C, and the reaction is carried out for 2 hours, and the sample is controlled by gas chromatography, and the content of 2,3-dimethylaniline is less than 0.2 %, stop the reaction. The reaction process can be represented by the following reaction equation:
[0042]
[0043] The reaction material was cooled to 25°C, filtered, and the residue was washed twice with dimethyl disulfide, and the amount of dimethyl disulfide was 10 g each time. The organic phases...
Embodiment 3
[0045] In a 250mL four-necked bottle, add 0.64g (0.01mol, 99.5%) copper powder, 0.12g tetrabutylammonium iodide (Tetrabutylammonium iodide), 23.74g (0.25mol, 99%) dimethyl disulfide, stir , control the reaction temperature to be 10°C, add 13.01g (0.12mol, 95%) of n-butyl nitrite dropwise, the time for adding is 1.5 hours, after the addition, the temperature is raised to 40°C, and 12.22g (0.10mol, 99% %) 2,3-dimethylaniline, the dropping time is 1.5 hours, after the dropping is completed, the temperature is raised to 50°C, and the reaction is carried out for 4 hours, the sample is controlled by gas chromatography, the content of 2,3-dimethylaniline is less than 0.2%, Stop responding. The reaction process can be represented by the following reaction equation:
[0046]
[0047] The reaction material was cooled to 25°C, filtered, and the residue was washed twice with dimethyl disulfide, and the amount of dimethyl disulfide was 10 g each time. The organic phases were combined a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com