Cytochrome P450 119 and mutant and purpose thereof
A CYP119, 1.CYP119 technology, applied in the field of CYP119 enzymes and their mutants, can solve the problems of catalyzing thioether substrates that have not been reported, and achieve excellent economic benefits, high catalytic efficiency, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Example 1 Construction and expression of CYP119 mutant enzyme
[0041] 1. Construction of CYP119 enzyme mutants: according to the pET30a-CYP119 vector, the Quickchange Lighting Site-directed Mutagenesis Kit (Agilent Technologies) was used for point mutation. The PCR product was double digested and ligated to the pET30a vector to construct the pET30a-CYP119 mutant plasmid. This plasmid was transformed into Escherichia coli BL21(DE3)plysS. Positive clones were selected and sequenced to verify the correctness of the mutant plasmid.
[0042] 2. Expression of wild-type and T213G+F1533G mutants: Inoculate the positive plasmid in 2 mL double-antibody LB liquid medium, and culture overnight at 37°C with shaking. Take 100 μL of the overnight cultured bacterial solution in 50 mL of double-antibody TB medium. Add 250 μL / LTrace Element, culture at 37°C with shaking until OD 0.6. Add 0.4mmol·L -1 IPTG, 32°C, induced for 24h. 12000rpm, centrifuge at 4°C for 10min, discard the su...
Embodiment 2
[0044] Example 2 Screening of reaction conditions for CYP119 wild-type and mutant enzymes to catalyze the oxidation of thioanisole
[0045] In this embodiment, screening is carried out for the concentration of TBHP, and the screening conditions are shown in Table 1.
[0046] Table 1 The results of CYP119 wild-type enzyme catalyzing the reaction of thioanisole at different TBHP concentrations at 35°C and pH 7.5
[0047]
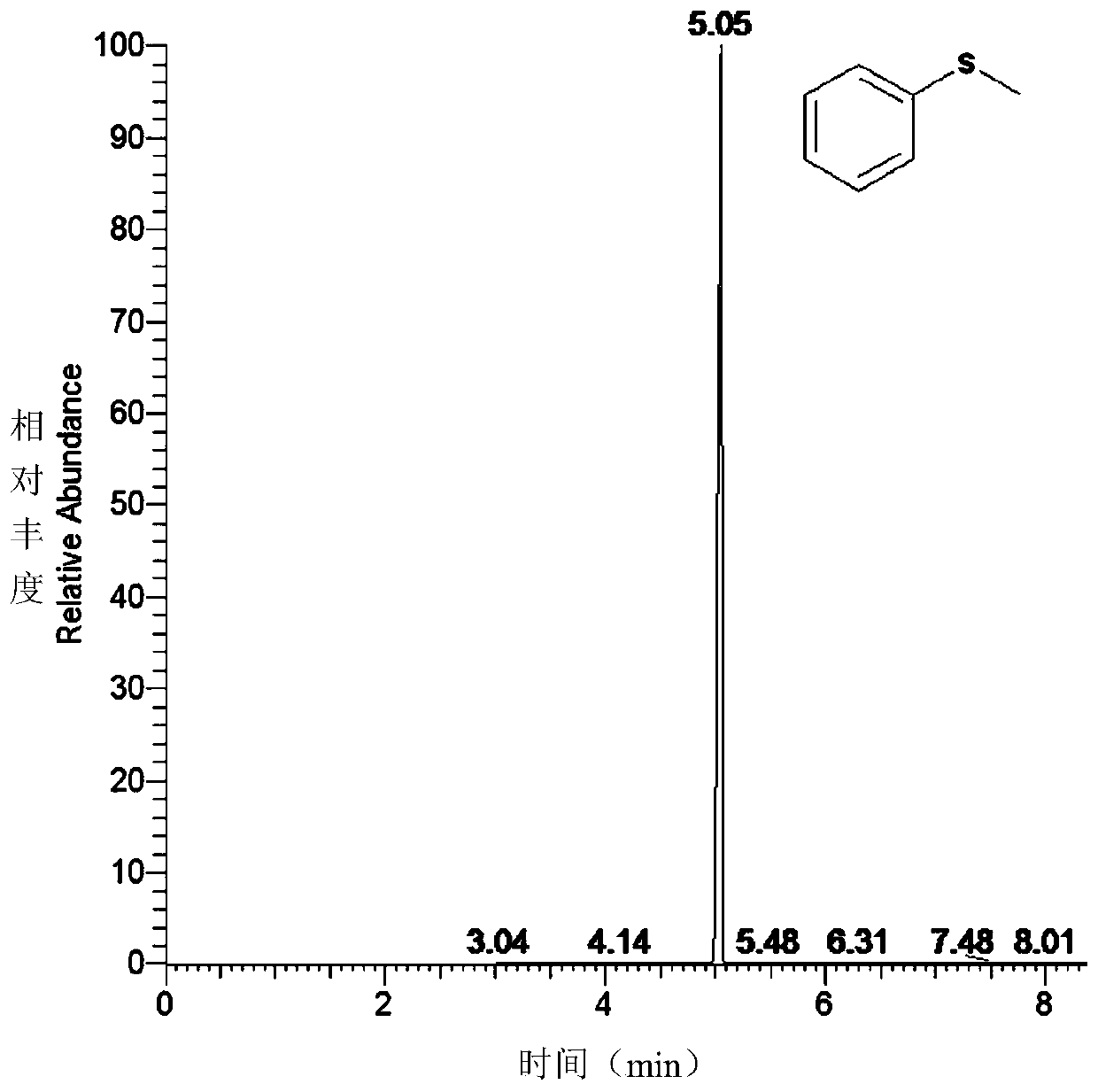
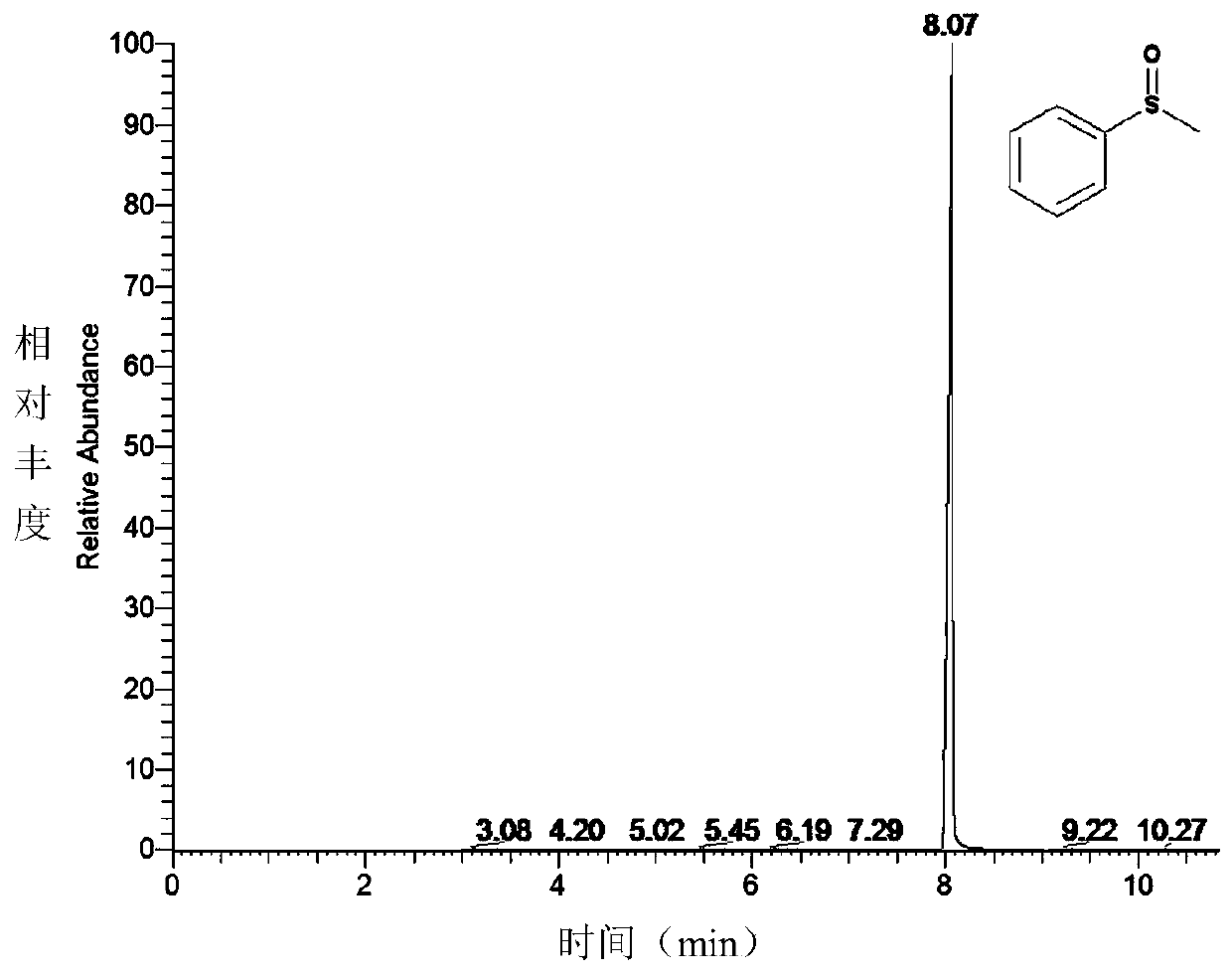
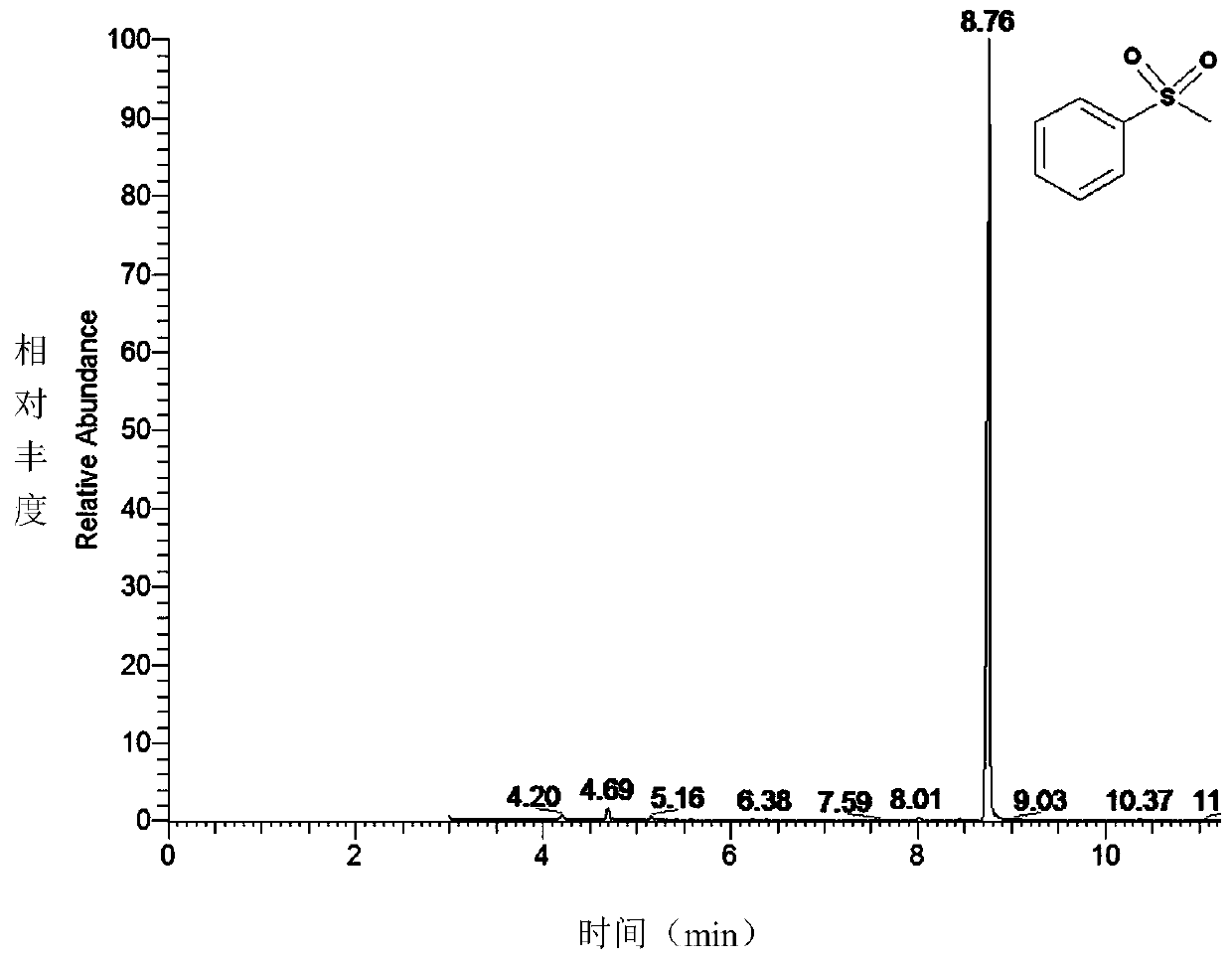
[0048] This embodiment explores the concentration of the nutrient TBHP, and finally determines the optimal condition: the amount of TBHP is 10 times that of the substrate concentration (in this embodiment, the substrate concentration is 2mmol L -1 ), 35°C, pH 7.5. GC detection results of CYP119 wild-type enzyme catalyzed thioanisole oxidation reaction Figures 1A-1E .
Embodiment 3
[0049] Example 3 CYP119 wild-type enzyme catalyzes the oxidation reaction of thioanisole and the effect verification of thioanisole respectively
[0050] Reaction conditions: temperature 35°C, substrate sulfide anisole 2mmol·L -1 , Enzyme 7 μmol L -1 . The reaction buffer system is 50mmol·L -1 bis-Tris buffer, pH 7.5, total system 200μL. After preheating at 35°C for 10 min, add TBHP (20 mmol L -1 ) to initiate the reaction. See Figures 1A-1E .
[0051] After 1 hour, with 200 μL CH 2 Cl 2 Quench the reaction, add 1.5 μL of 0.25 mol of acetophenone, and extract three times. The conversion of the substrate was checked by GC. GC-MS detection conditions: Thermo Scientific ITQ900, DB-1MS column (30m×0.25mm), 90°C for 2min; 90-210°C, 10°C / min to 210°C; 210°C for 1min. Under this condition, the wild-type CYP119 enzyme catalyzes the oxidation of thioanisole to benzyl sulfoxide and benzyl sulfone, and the yields are 59.3% for benzyl sulfoxide and 30.3% for benzyl sulfone.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com