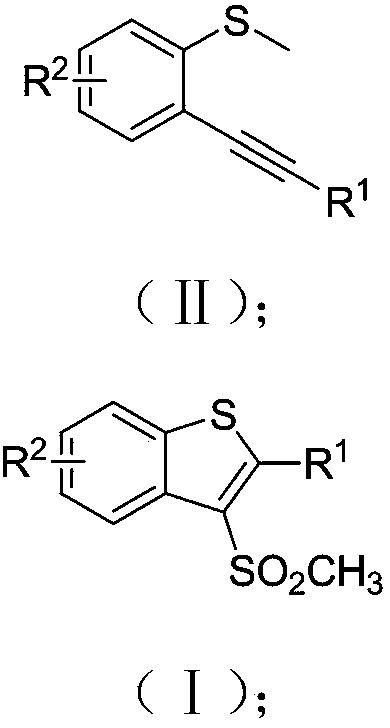
Preparation method of 3-methylsulfonyl-2-substituted benzothiophene compound
A technology of benzothiophene and methylsulfonyl, which is applied in the field of preparation of 3-methylsulfonyl-2-substituted benzothiophene compounds, can solve the problems of good economy of reaction atoms, achieve good application prospects, and avoid strong acid raw materials the effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
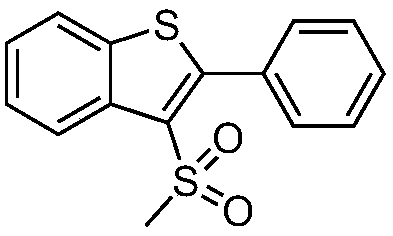
[0032] A preparation method of 3-methylsulfonyl-2-phenylbenzothiophene, the method is as follows:
[0033] Add 0.2mmol o-phenylynylanisole sulfide, 0.4mmol sodium metabisulfite, xmmol% sodium methylsulfinate and 2.0mmol% Ru(bpy) to the reaction tube sequentially. 3 Cl 2 , stop the reaction tube with a rubber stopper and place it in high-purity nitrogen or argon to replace the gas, so that the system is in anaerobic conditions, add 2mL of 1,2-dichloroethane, place it around a 35-watt fluorescent lamp and stir until complete reaction until. After the reaction, the reaction solution was directly concentrated under reduced pressure, and separated by column chromatography, using a mixture of petroleum ether and ethyl acetate as the mobile phase, and purified to obtain compound 1, namely 3-methylsulfonyl-2-phenylbenzo Thiophene, and by 1 H NMR, 13 C NMR and HRMS (ESI) confirmed its structure. The structure of compound 1 is characterized as: 1 H NMR (400MHz, CDCl 3...
Embodiment 2
[0036] A preparation method for 3-methylsulfonyl-2-phenylbenzothiophene, the compound prepared is the same as in Example 1, i.e. compound 1, the difference is that the photosensitizer used is different, and the photosensitizer used in the method is acid red 87, the details are as follows:
[0037]Add 0.2mmol o-phenylynylanisole sulfide, 0.4mmol sodium metabisulfite, 0.1mmol sodium methylsulfinate and 2.0mmol% acid red 87 (CAS No. 17372-87-1), stop the reaction tube with a rubber stopper and place it in high-purity nitrogen or argon to replace the gas, so that the system is in anaerobic condition, add 2mL of 1,2-dichloroethane, and place it at 35 watts Stir around fluorescent lamp until complete reaction.
[0038] After the reaction, the post-treatment process was the same as in Example 1, and the structural characterization of compound 1 was the same as in Example 1, and the yield was 86%.
Embodiment 3
[0040] A preparation method for 3-methylsulfonyl-2-phenylbenzothiophene, the compound prepared is the same as in Example 2, i.e. compound 1, the difference is that the photosensitizer used is different, and the photosensitizer used in the method is di[ 2-(2,4-Difluorophenyl)-5-trifluoromethylpyridine][2-2'-bi(4-tert-butylpyridine)]iridium di(hexafluorophosphate) salt (CAS No. 870987 -63-6), others are identical with embodiment 2, productive rate is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com