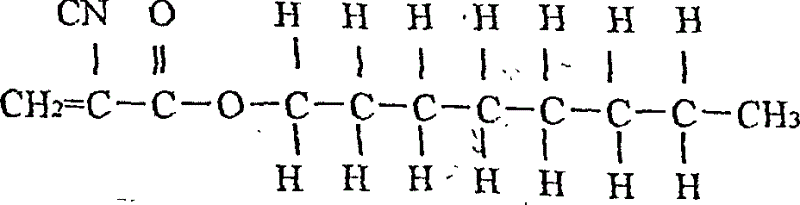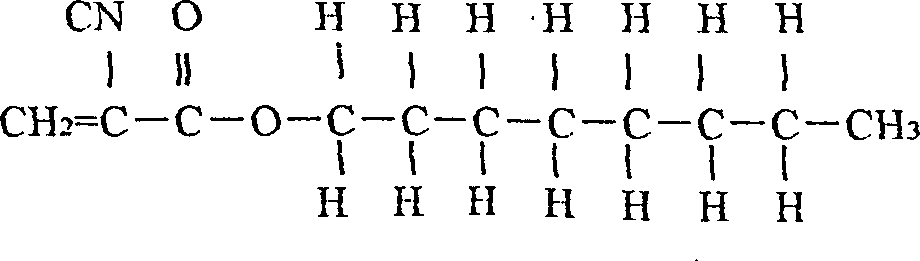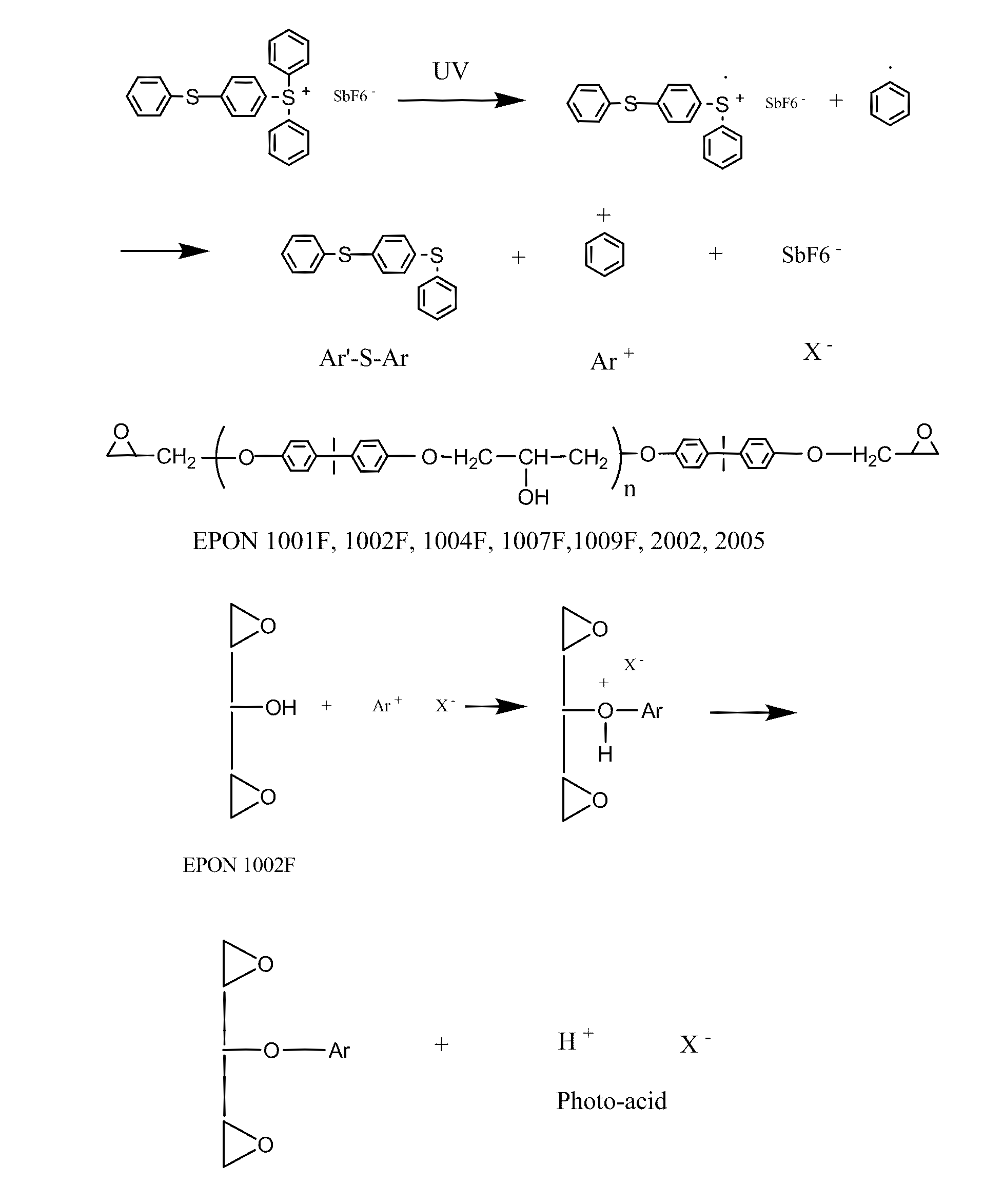Patents
Literature
105 results about "Biological Testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
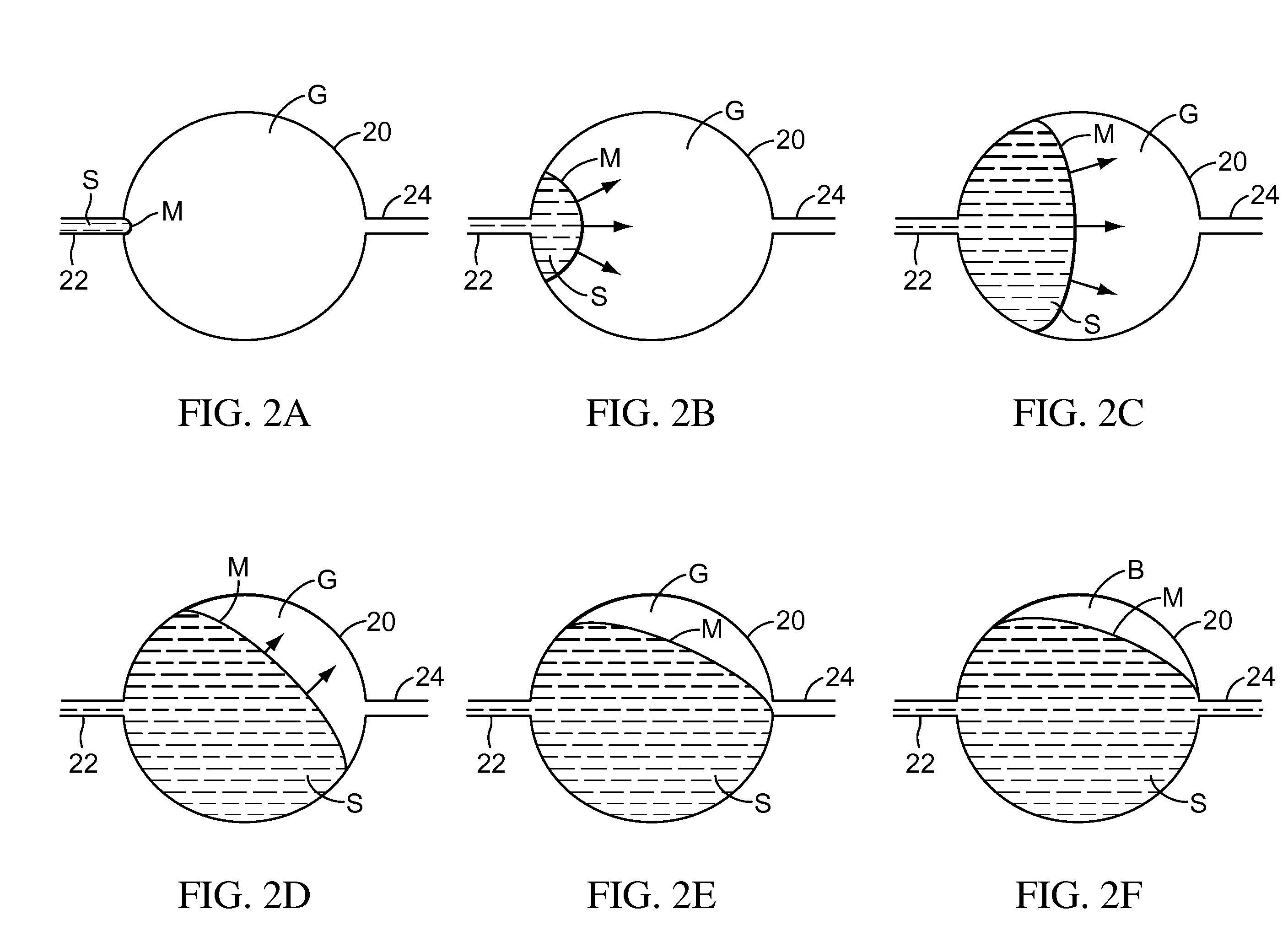
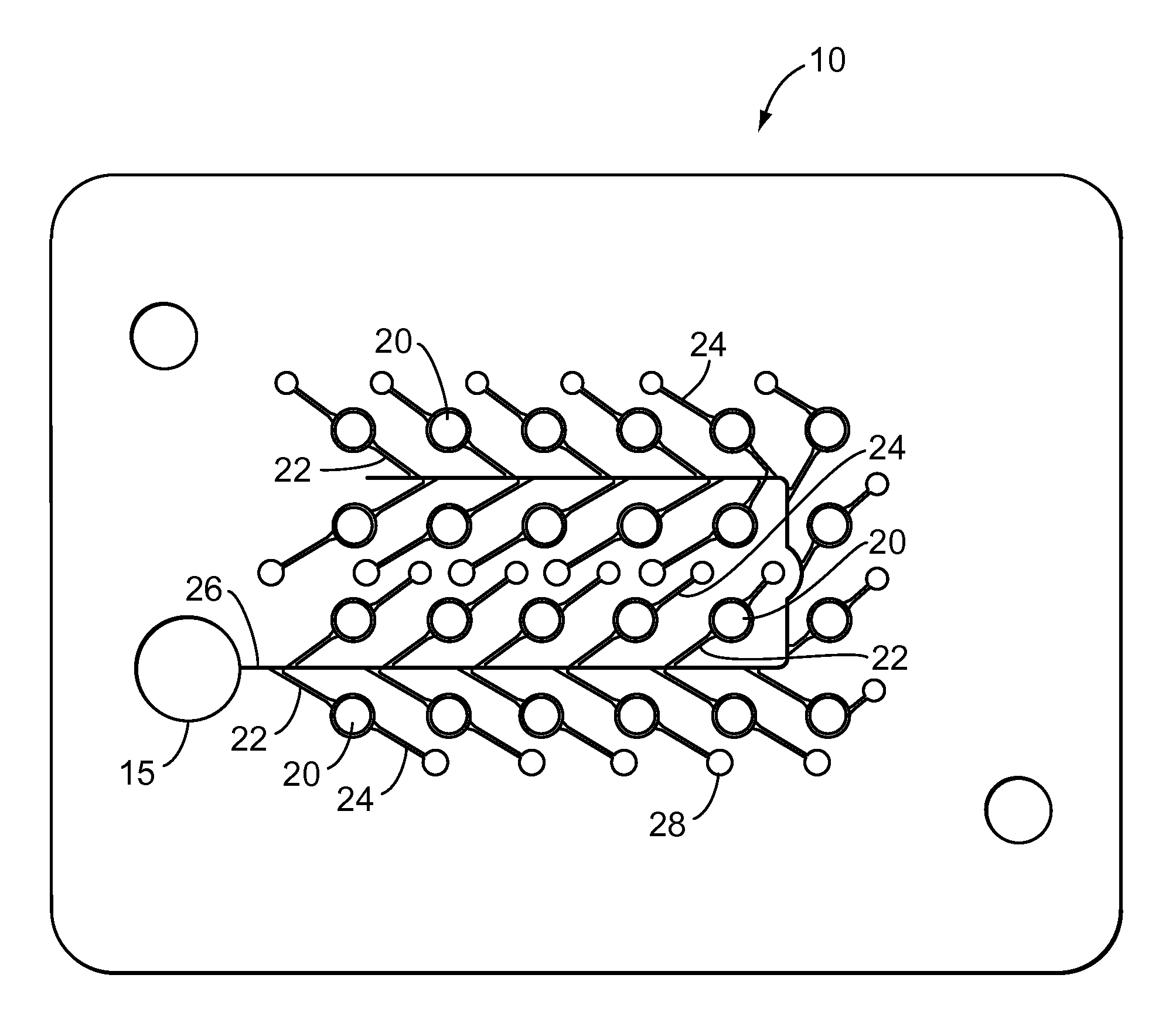
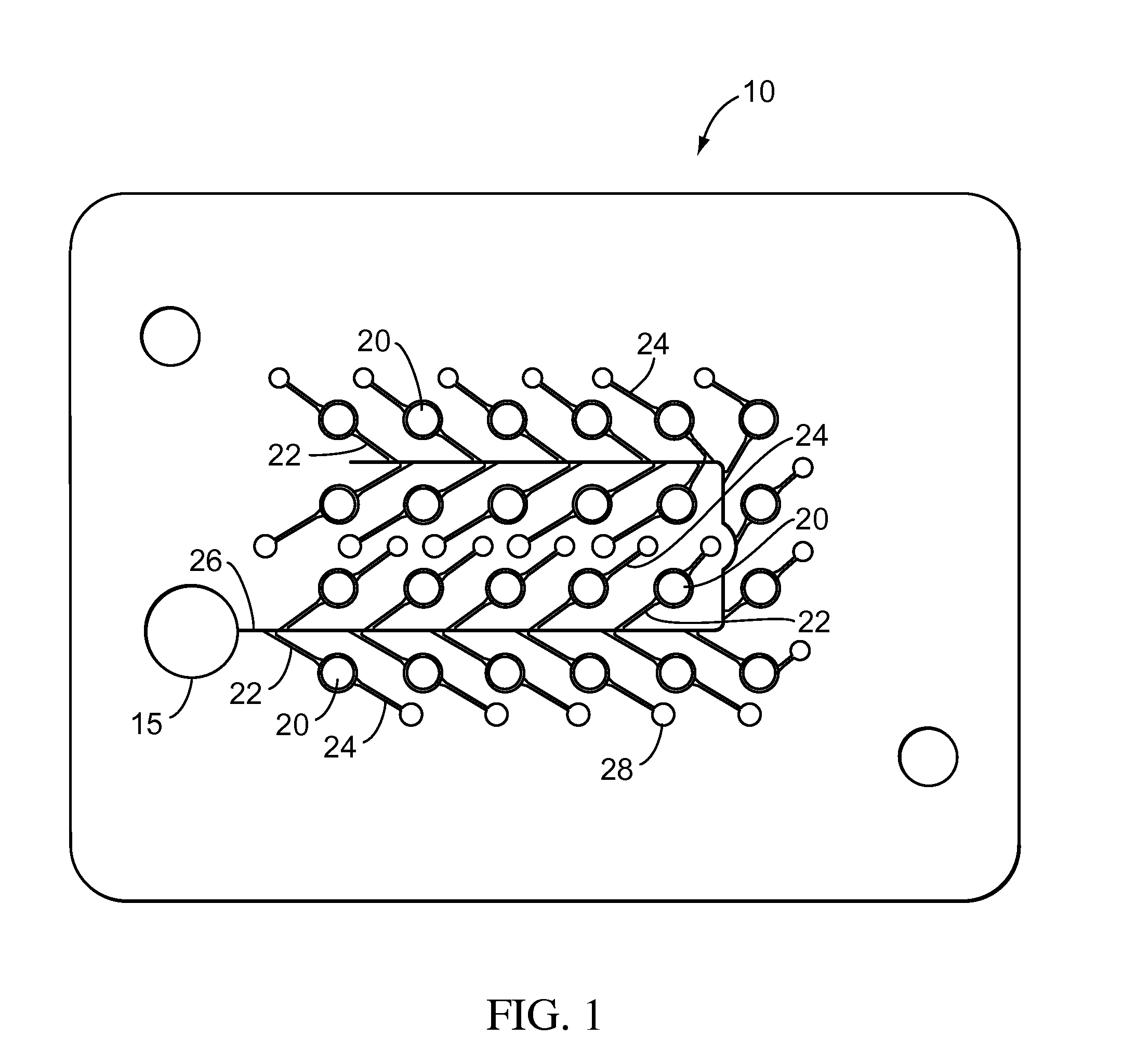
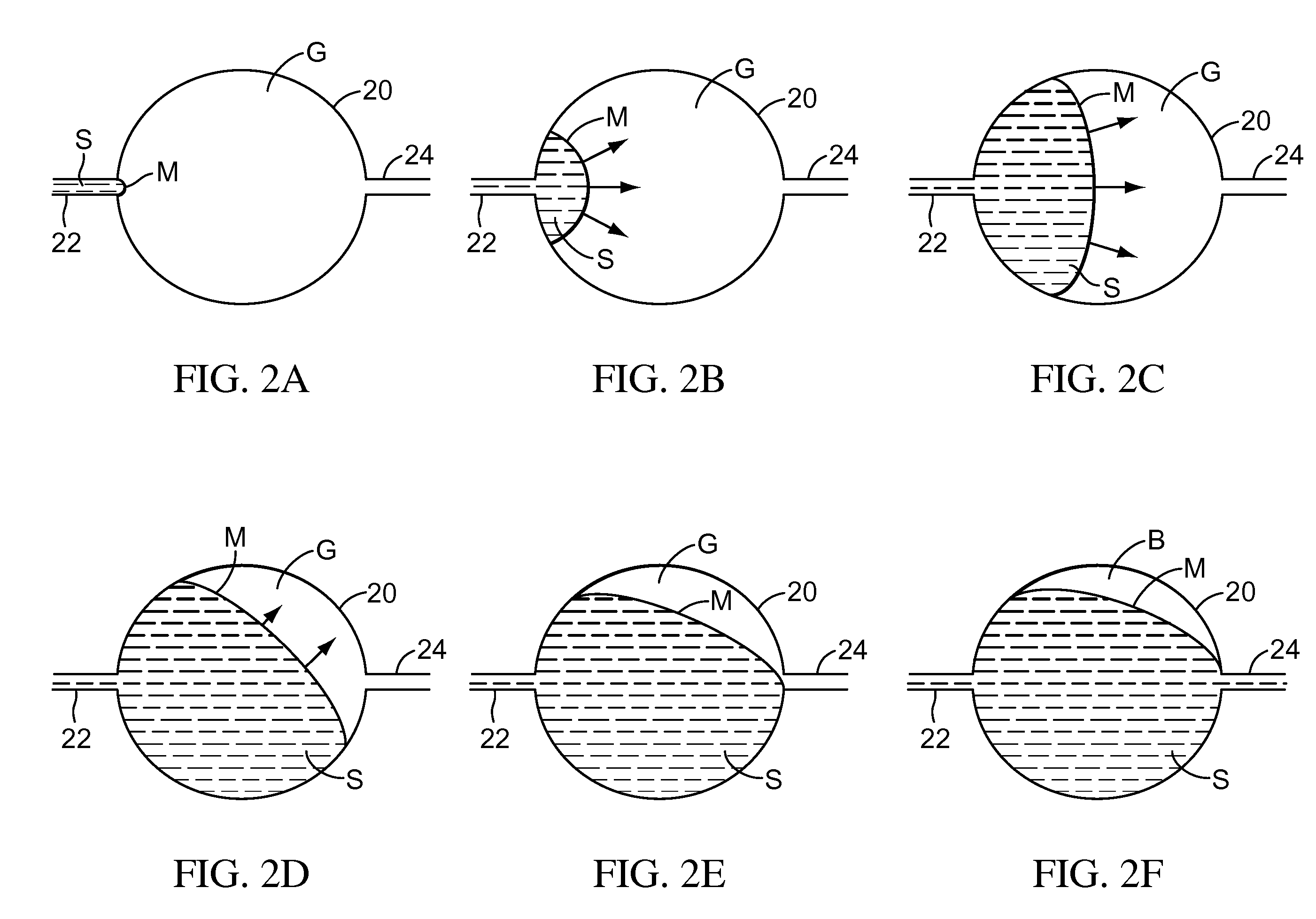
Testing performed to determine the effects of substances upon living organisms.
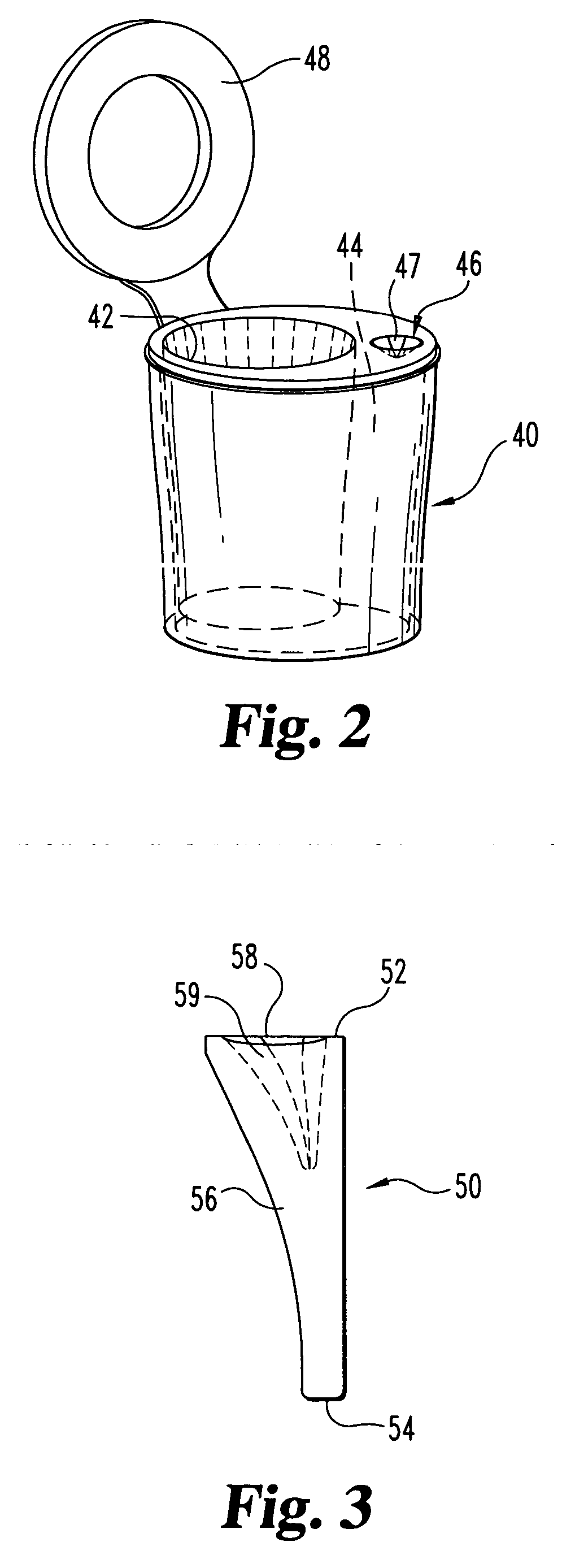
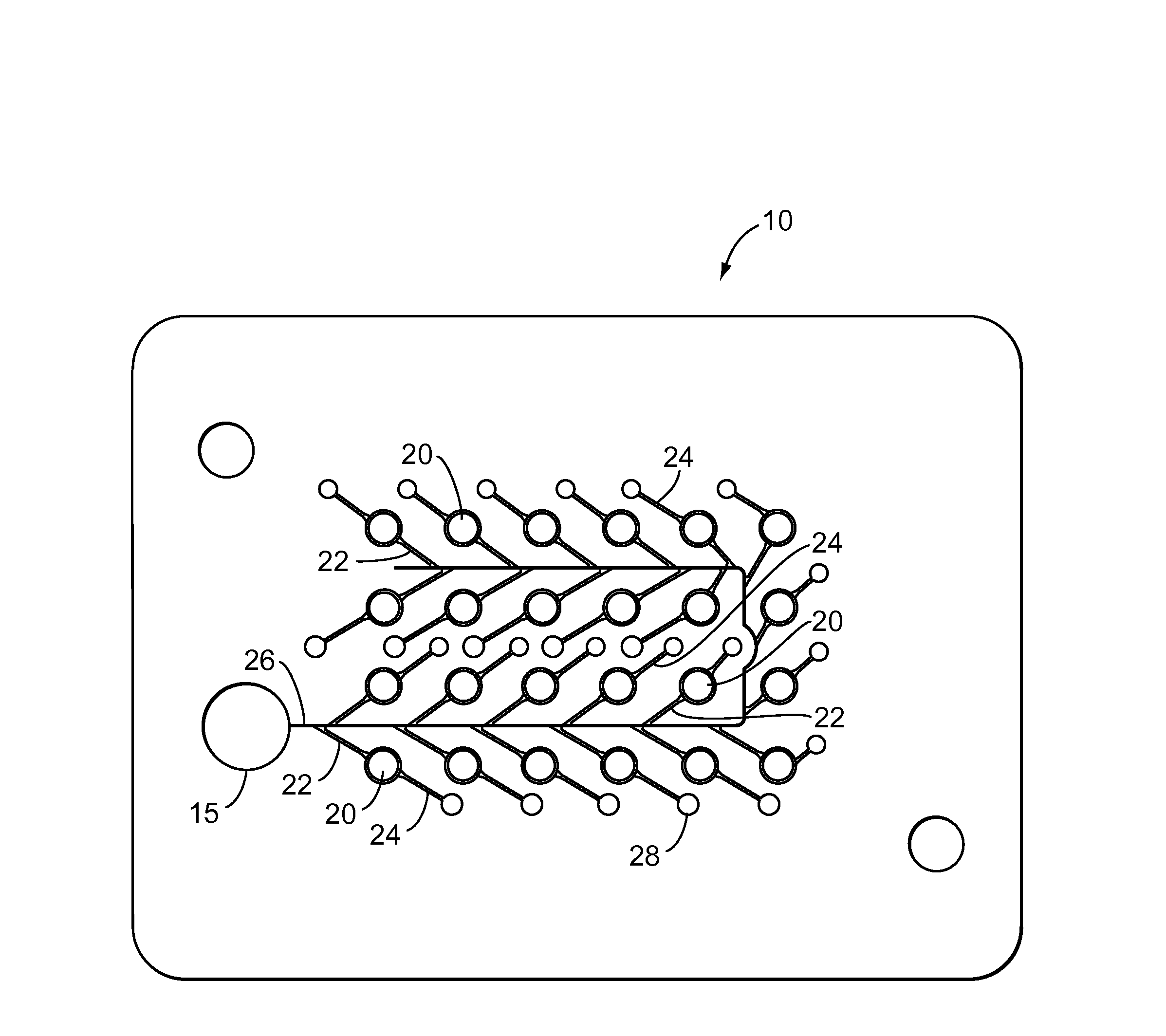
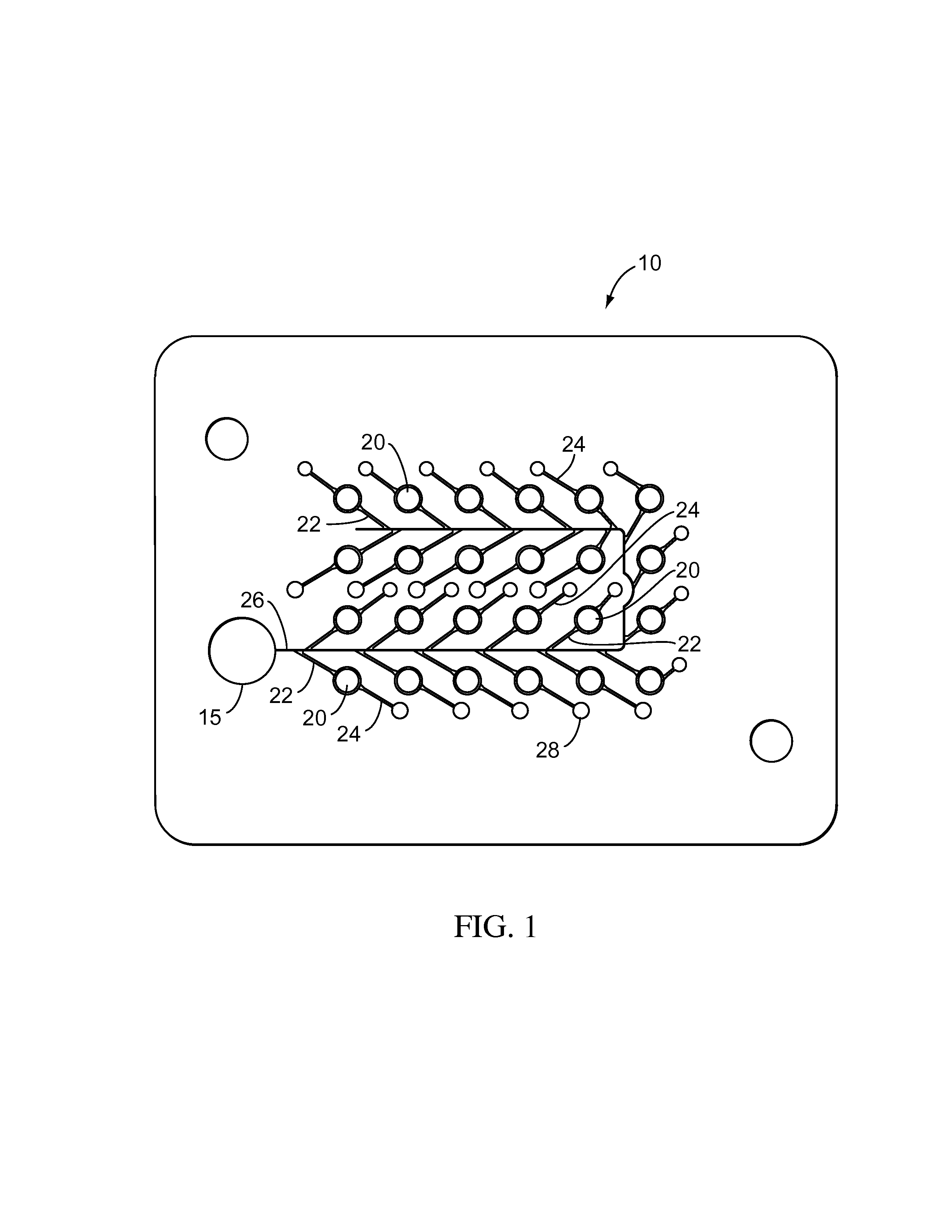
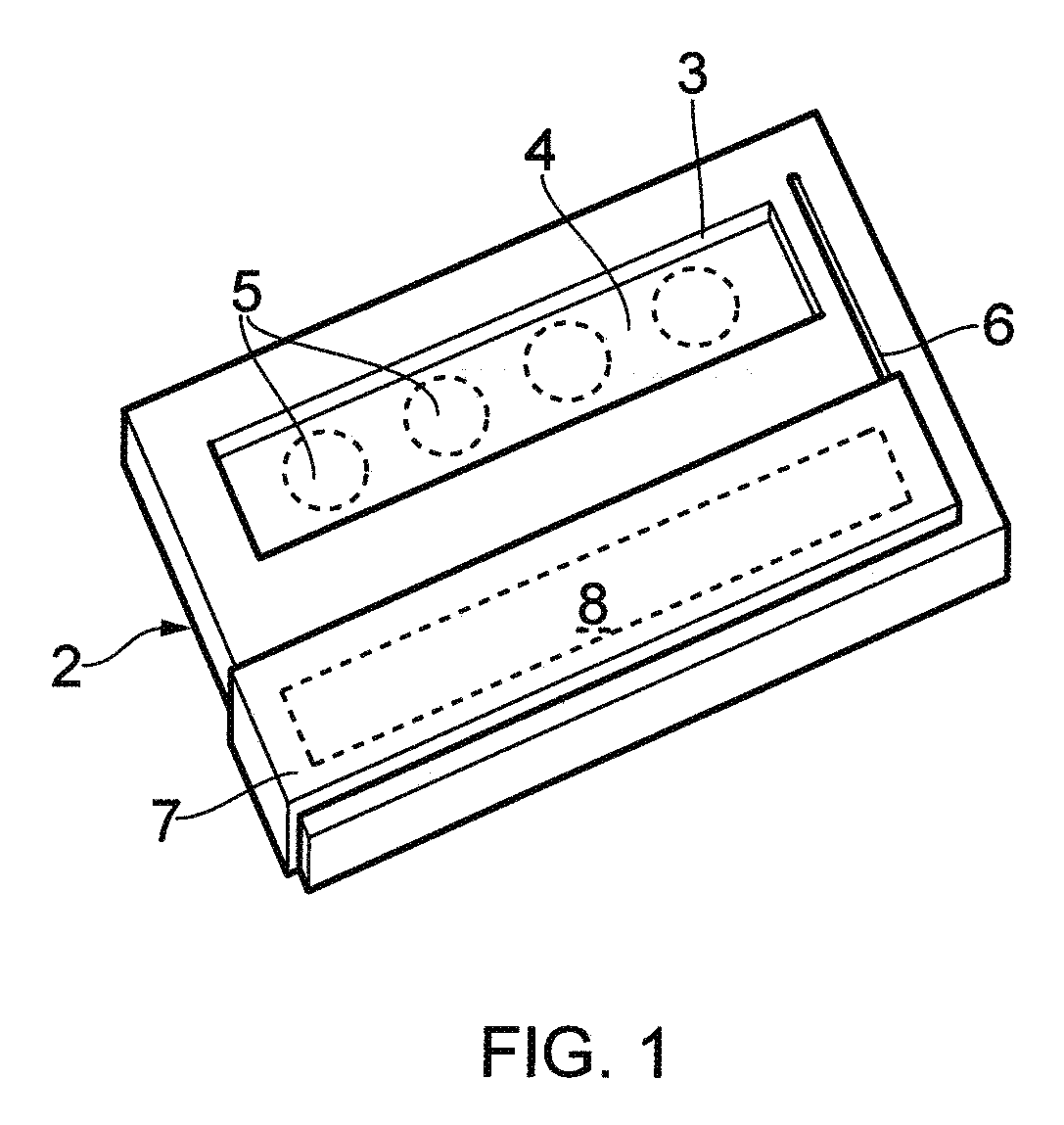
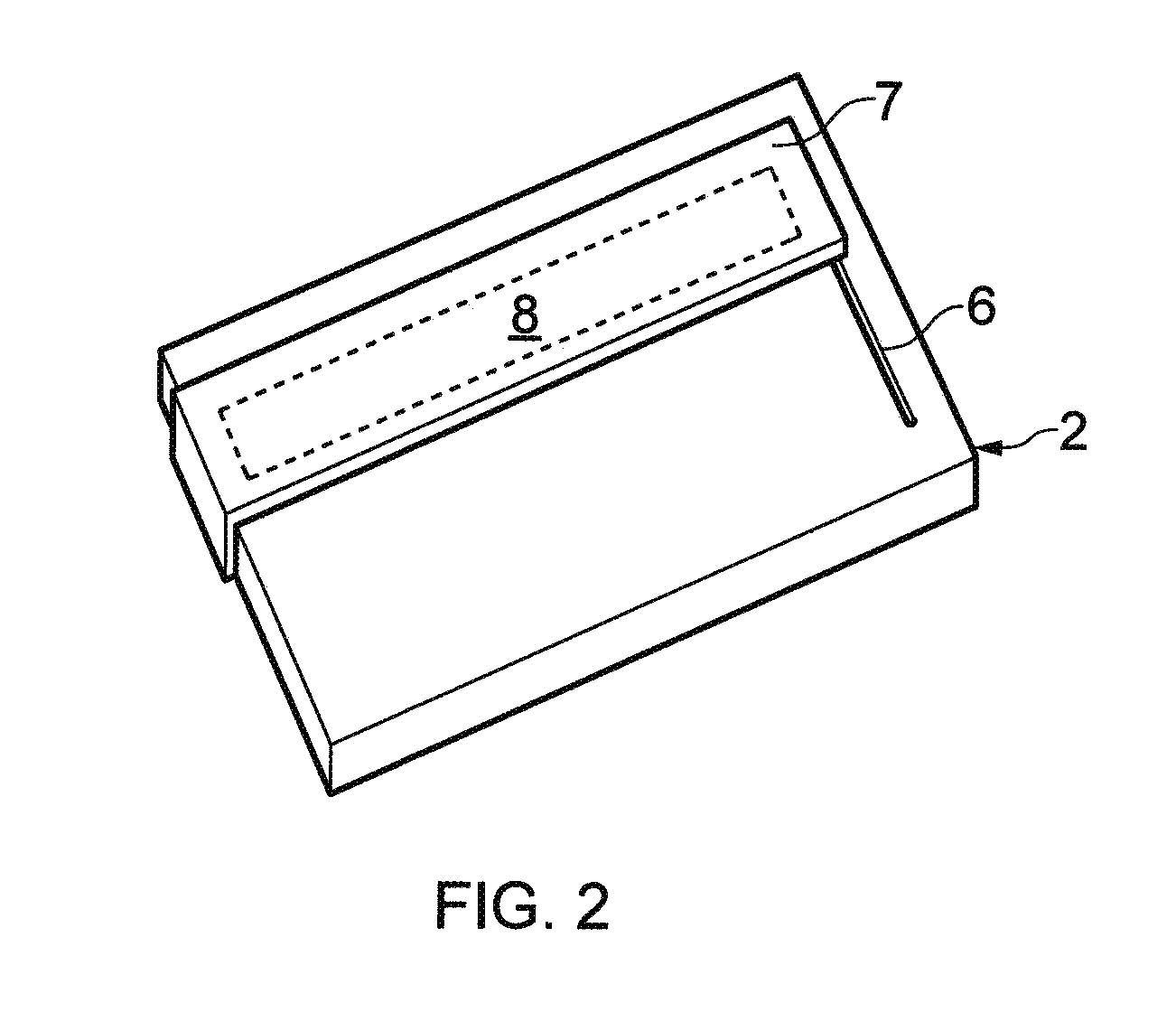
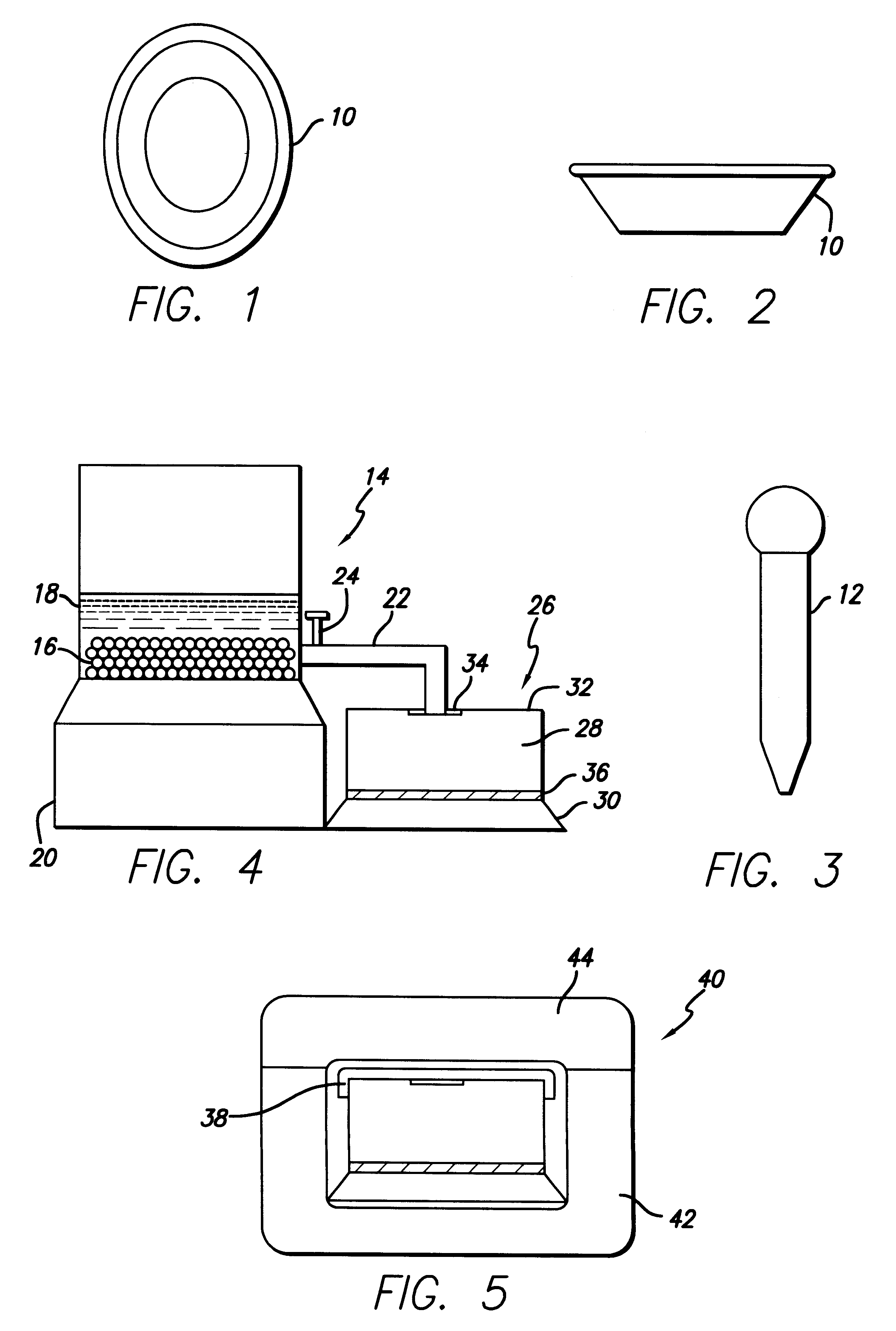
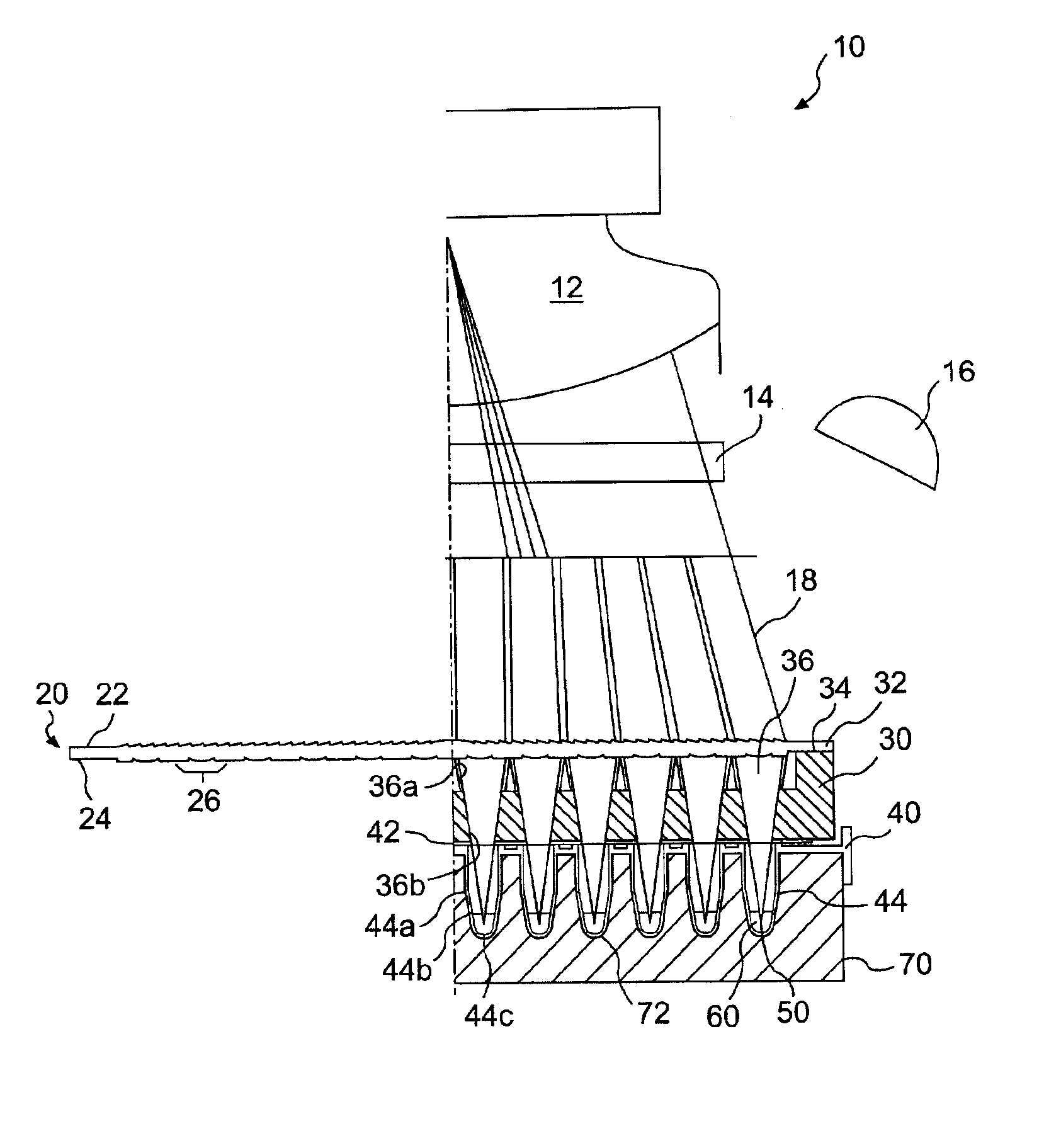
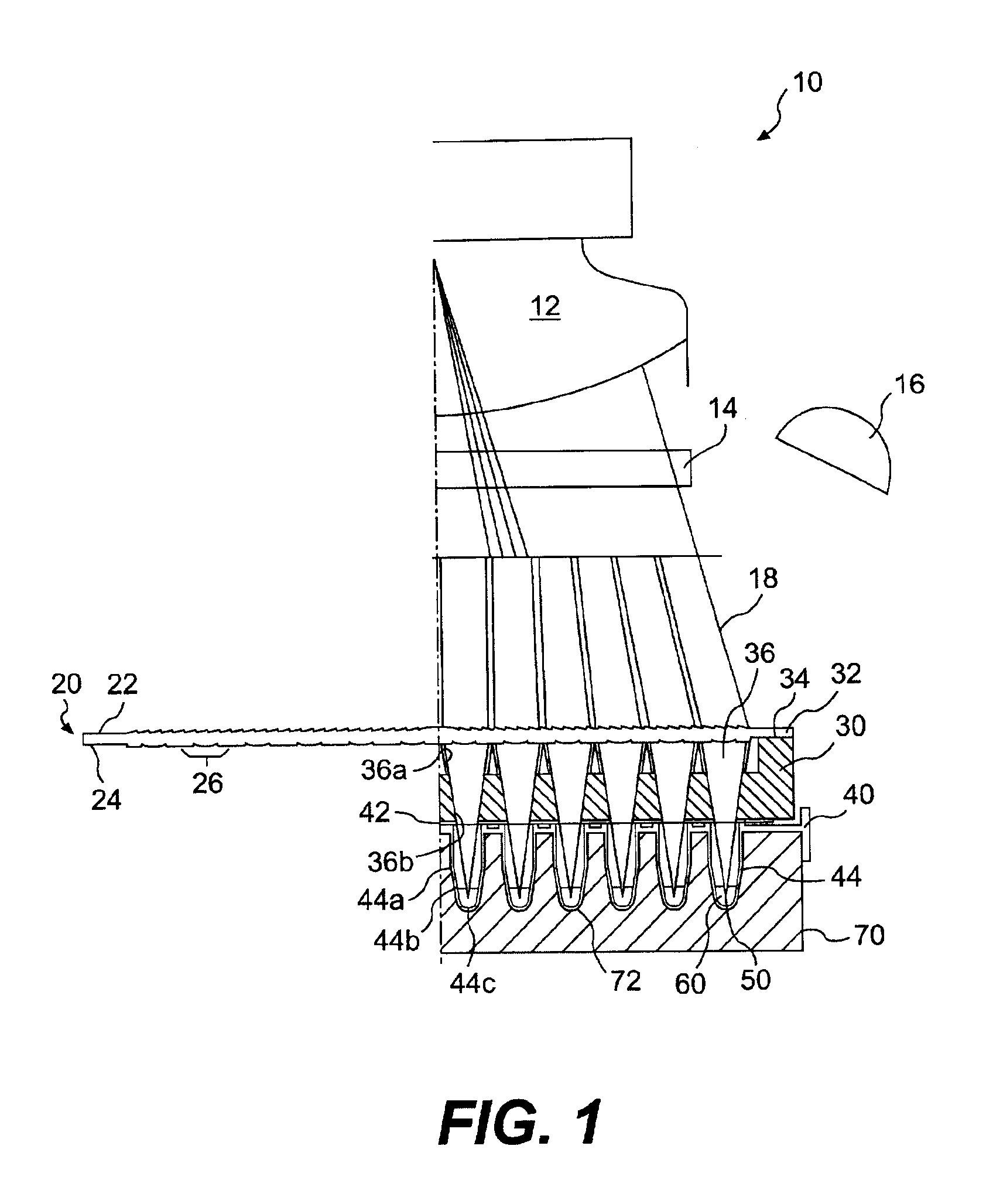
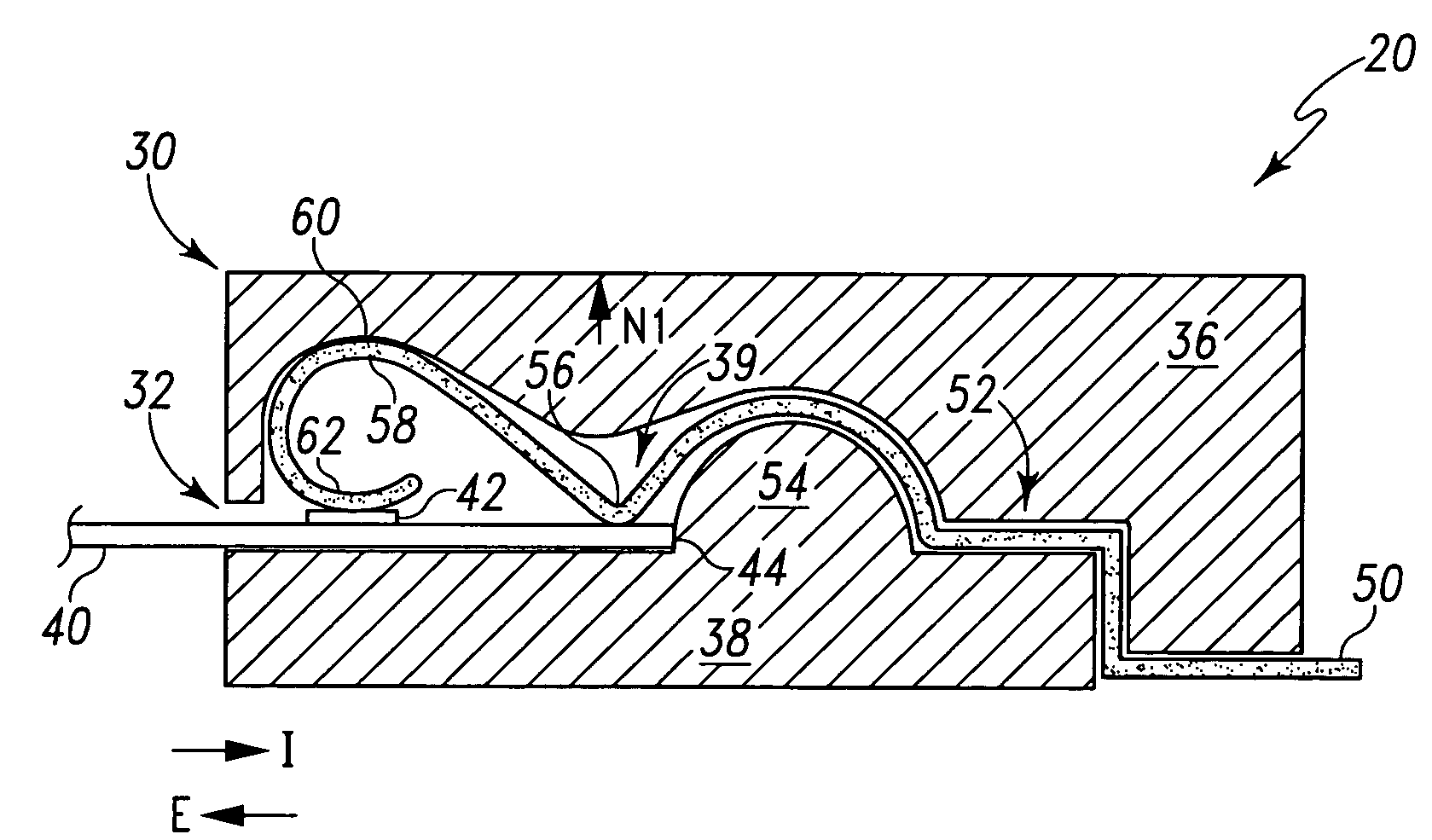
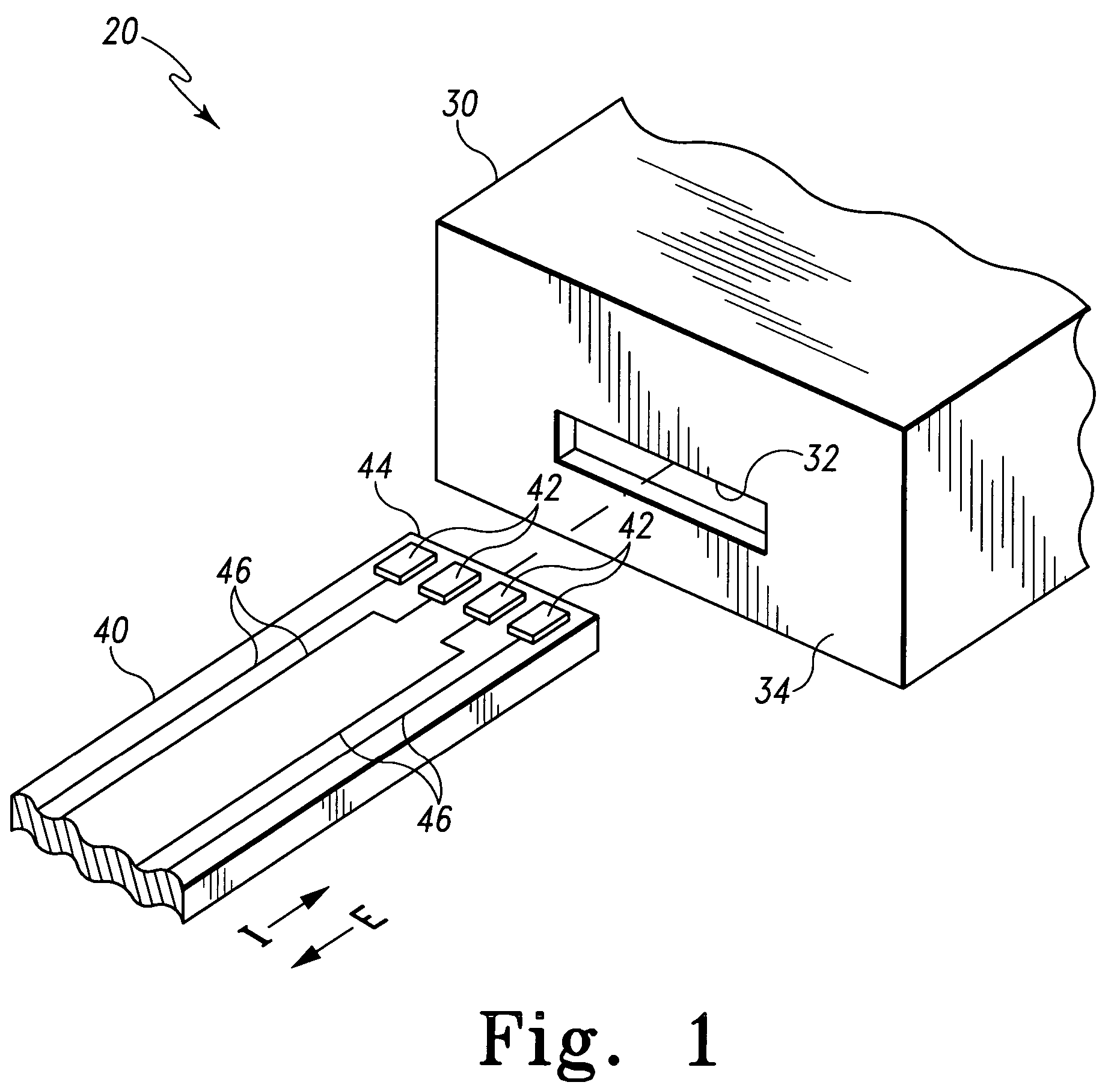
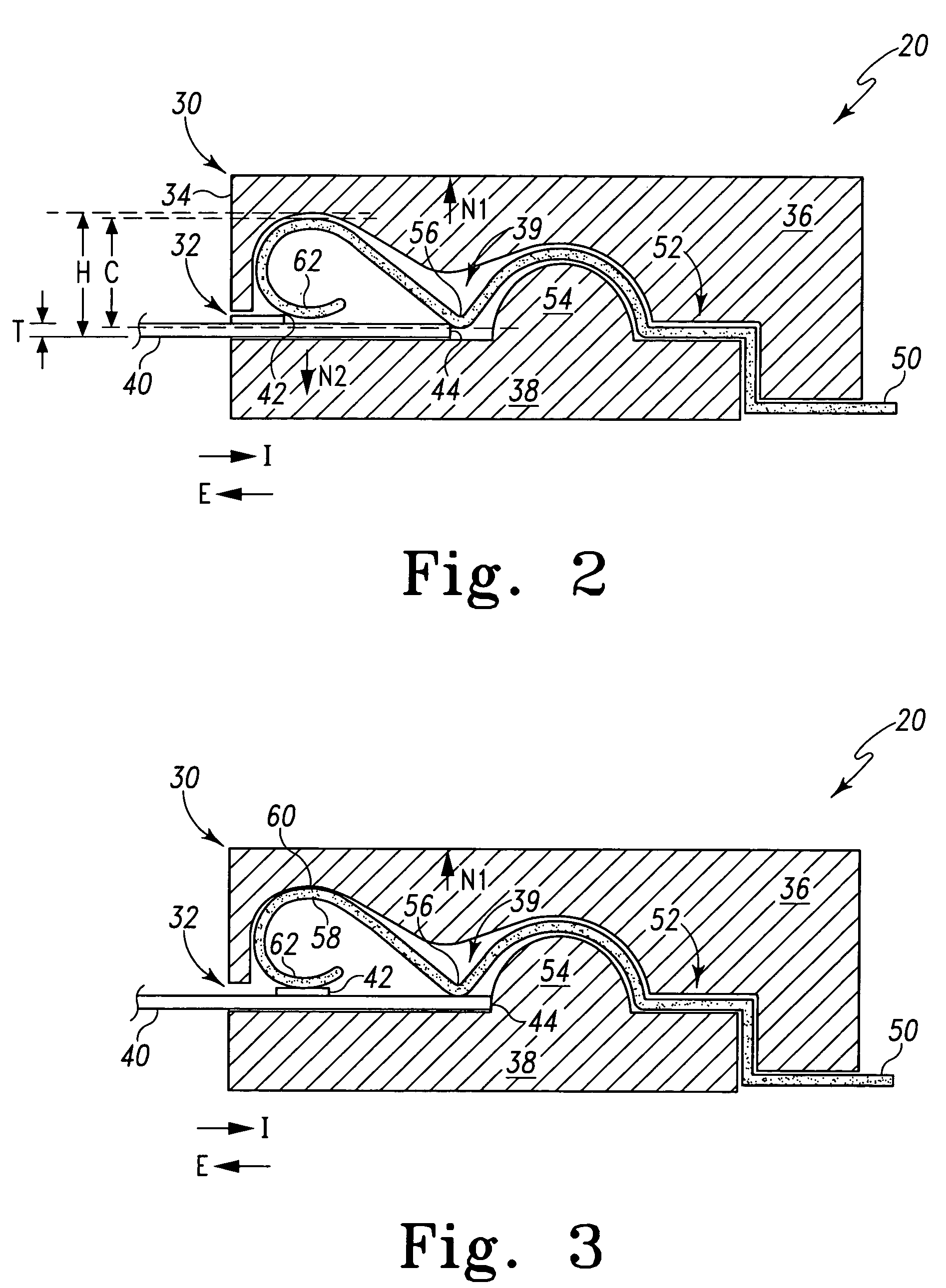
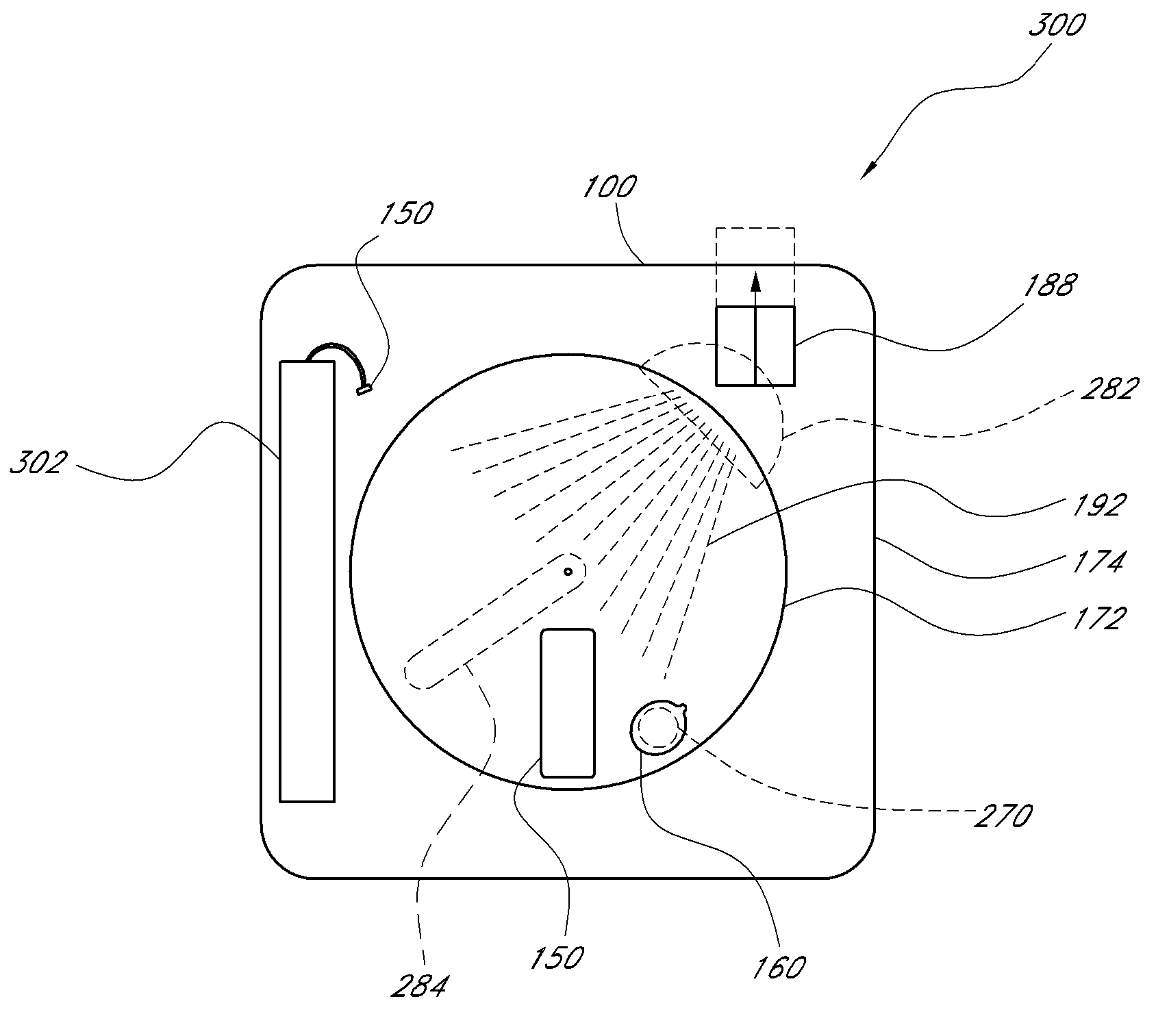
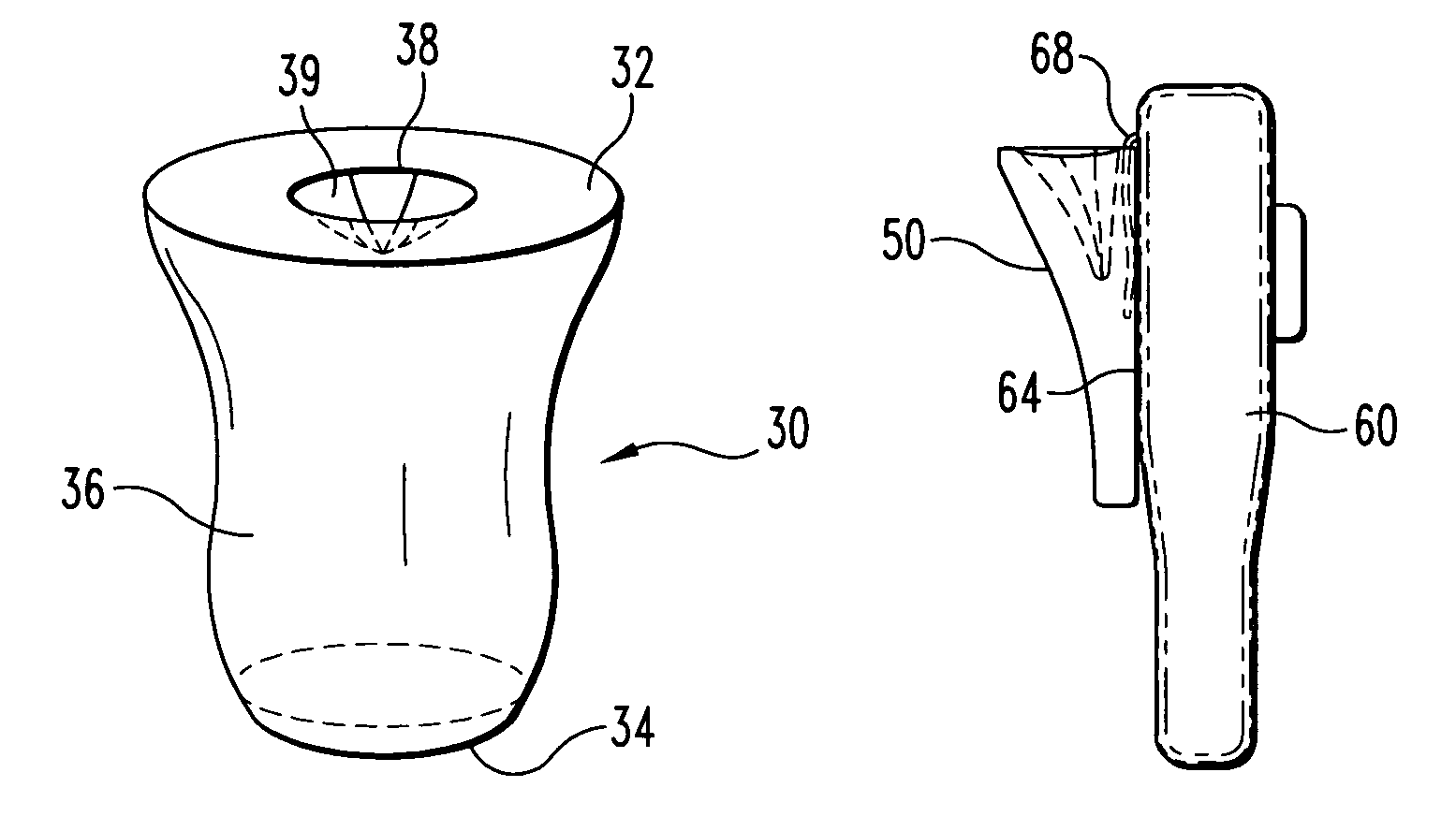
Biological testing system
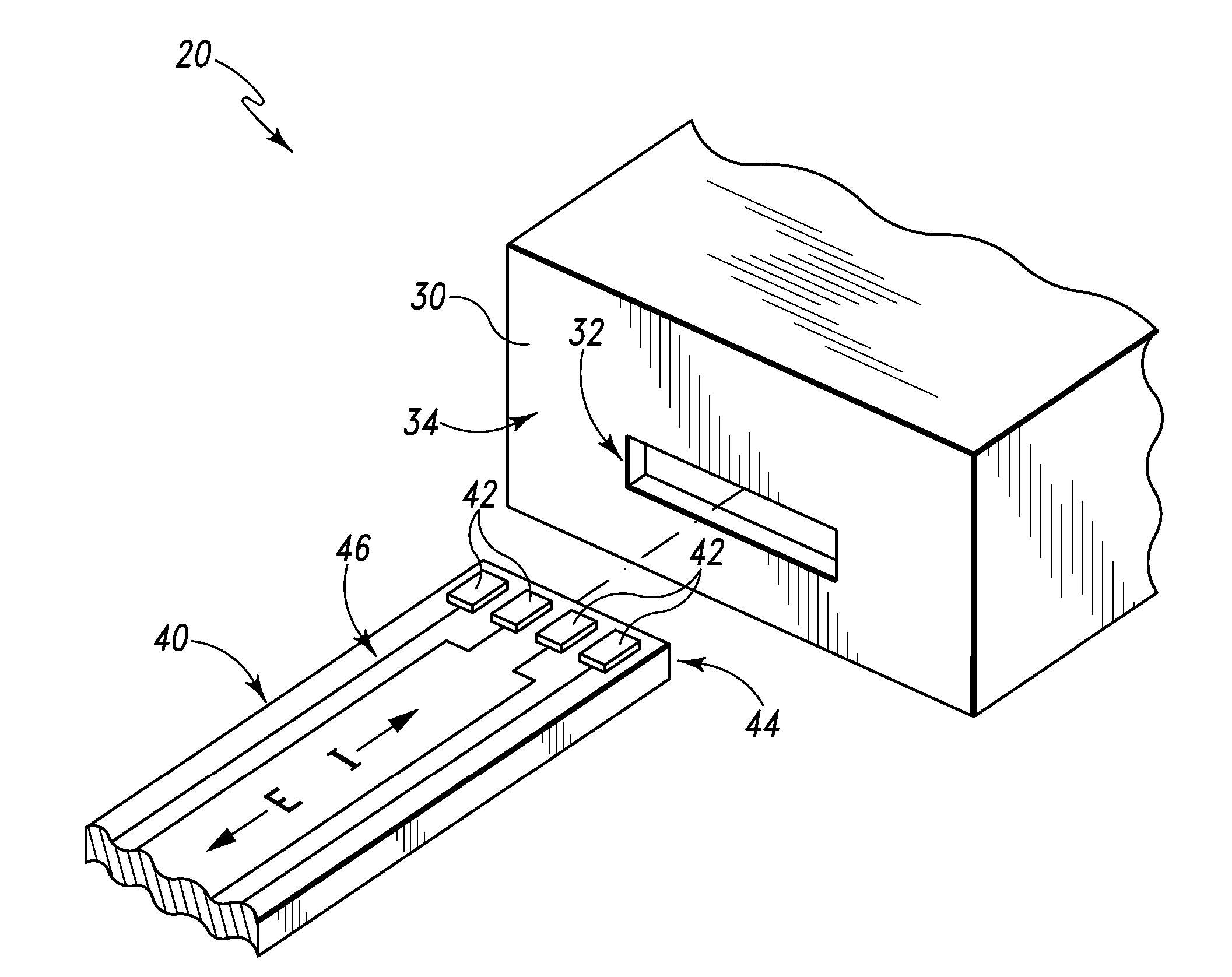
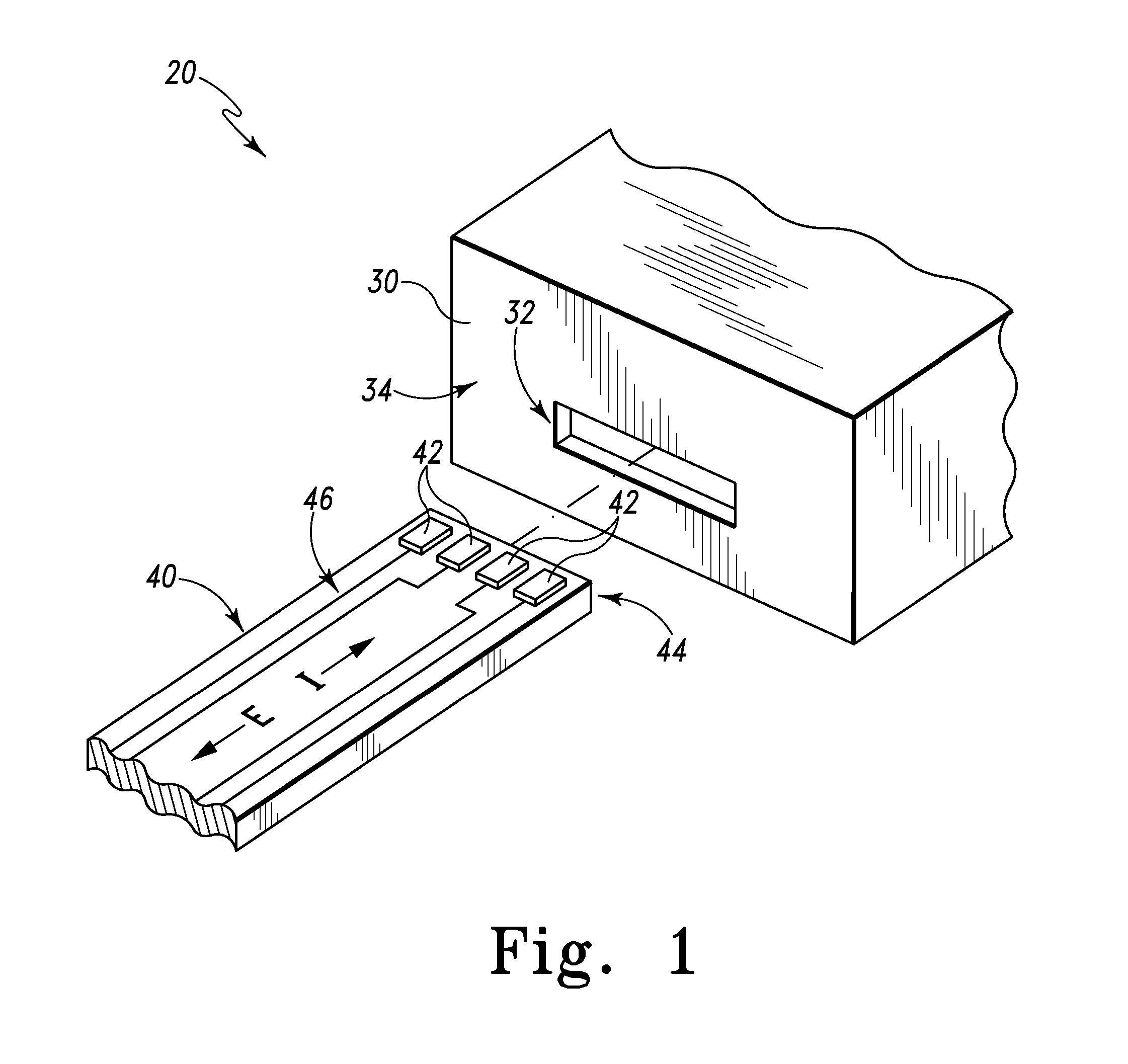
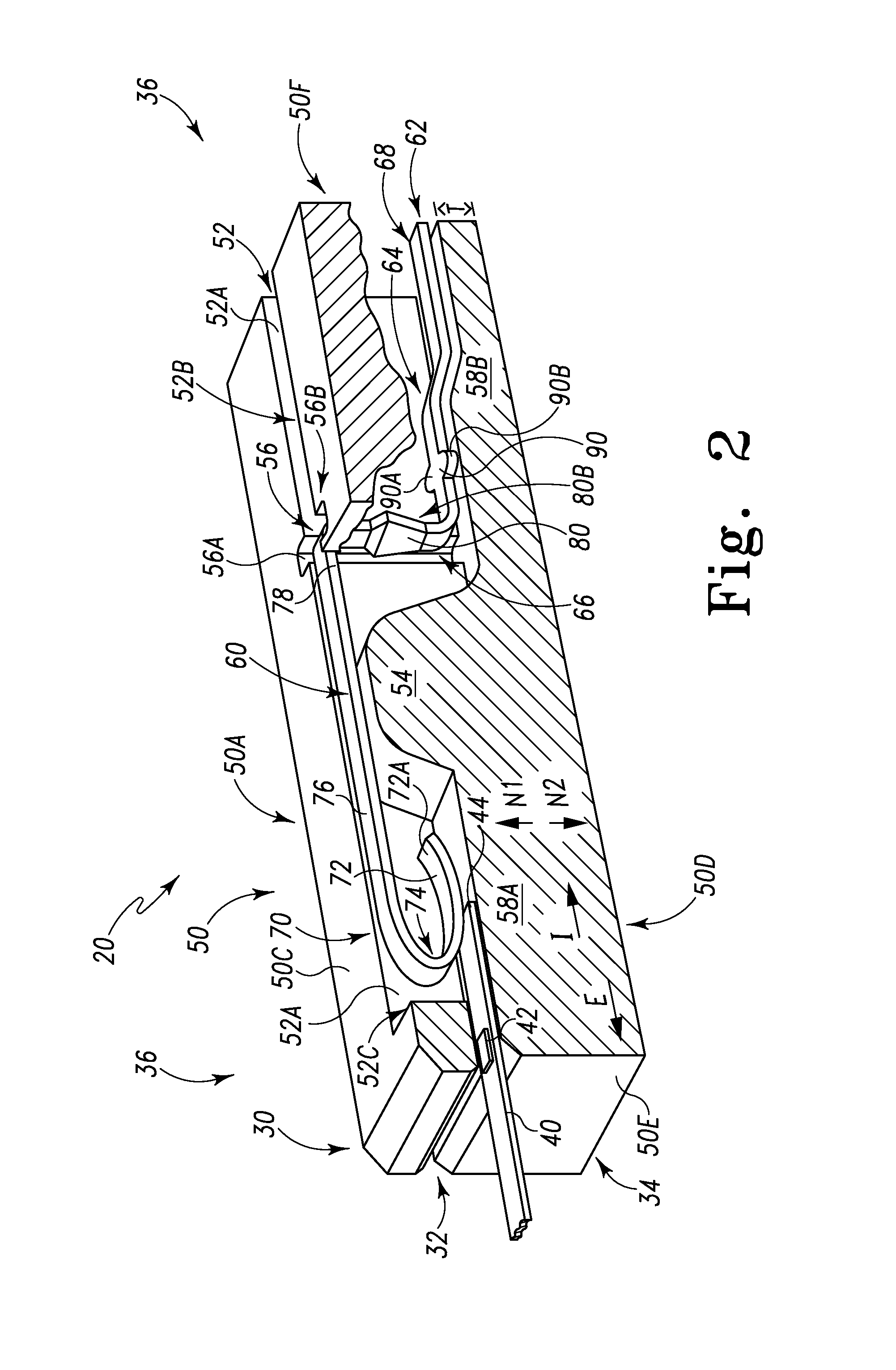
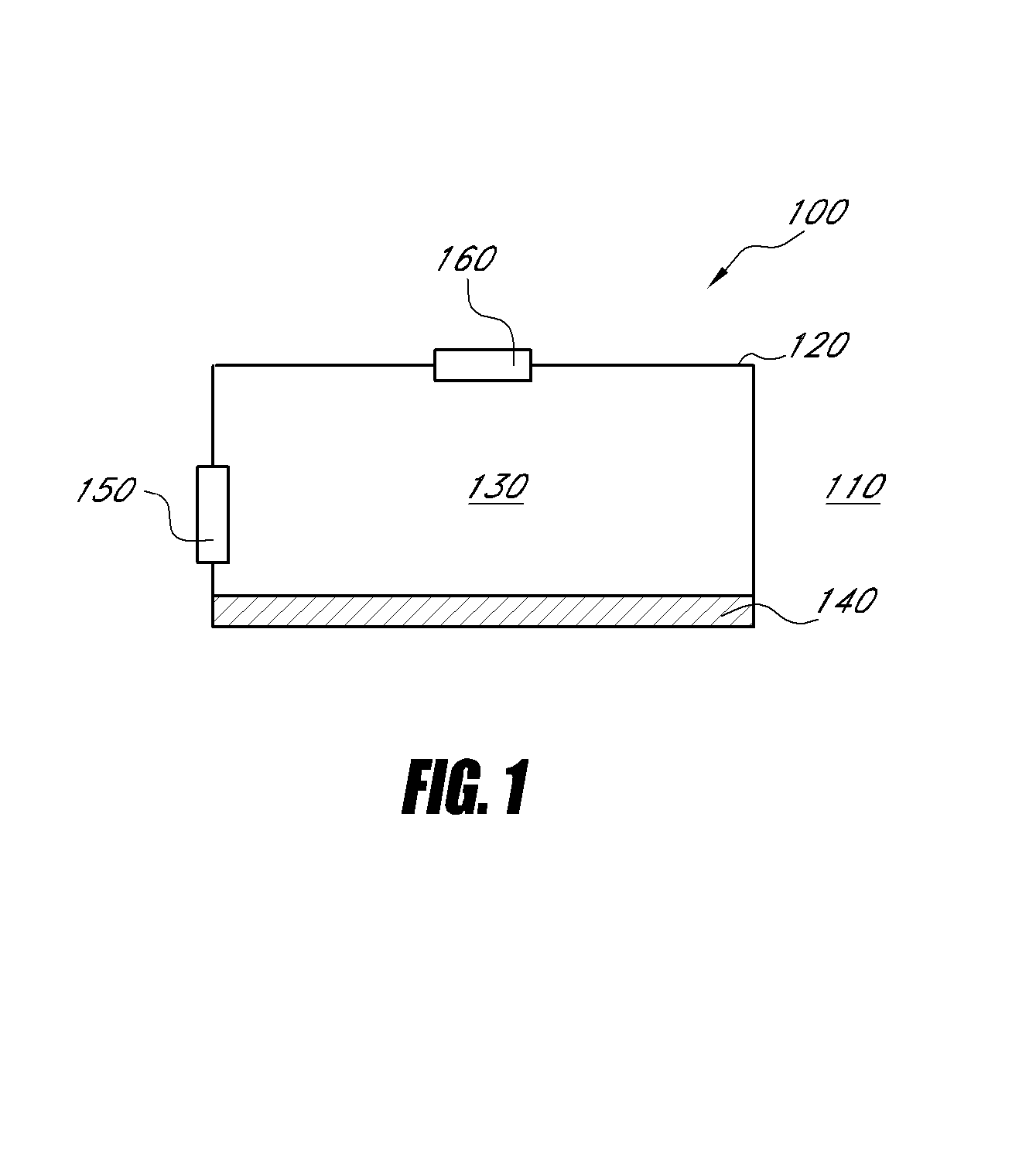
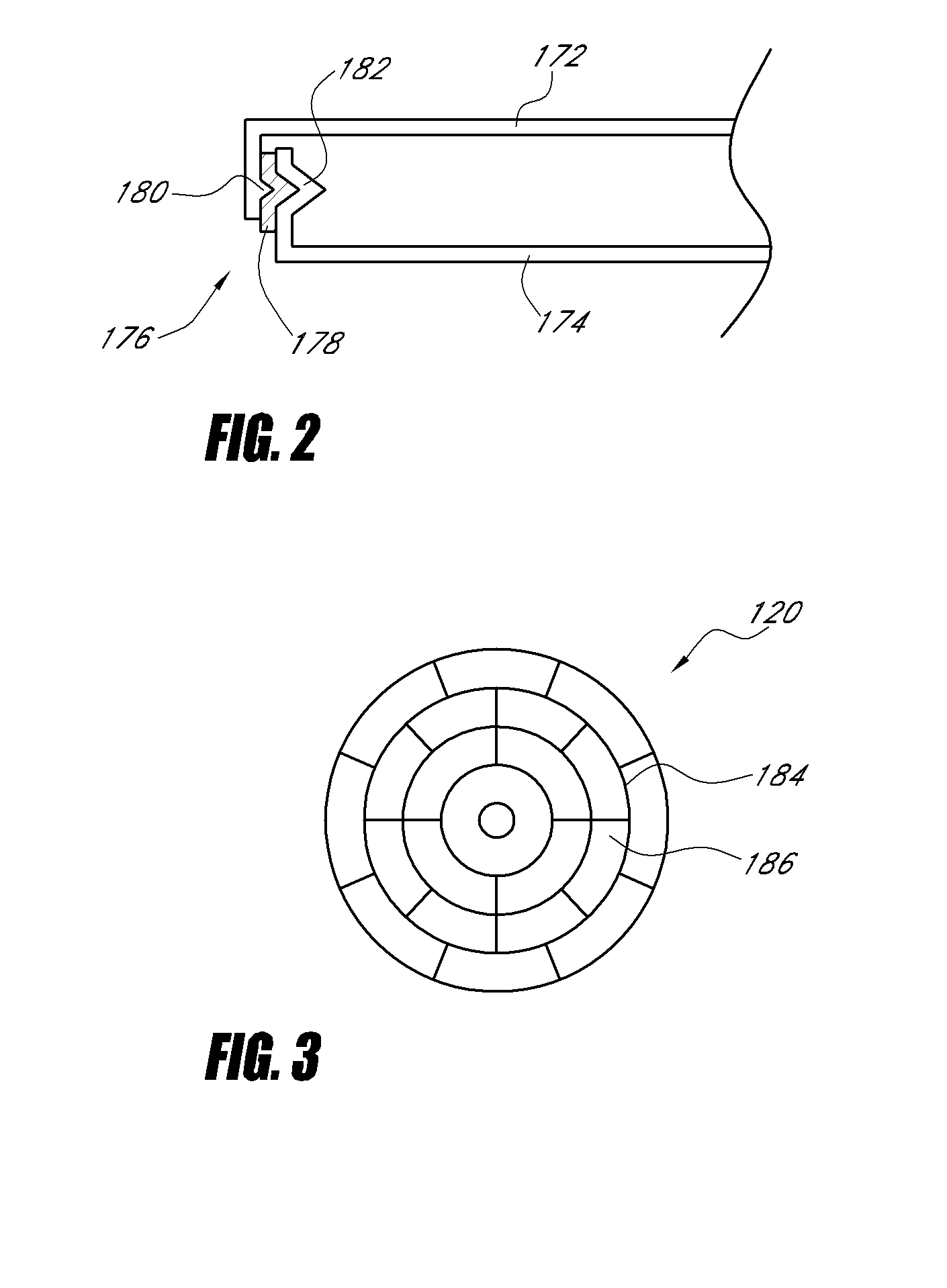
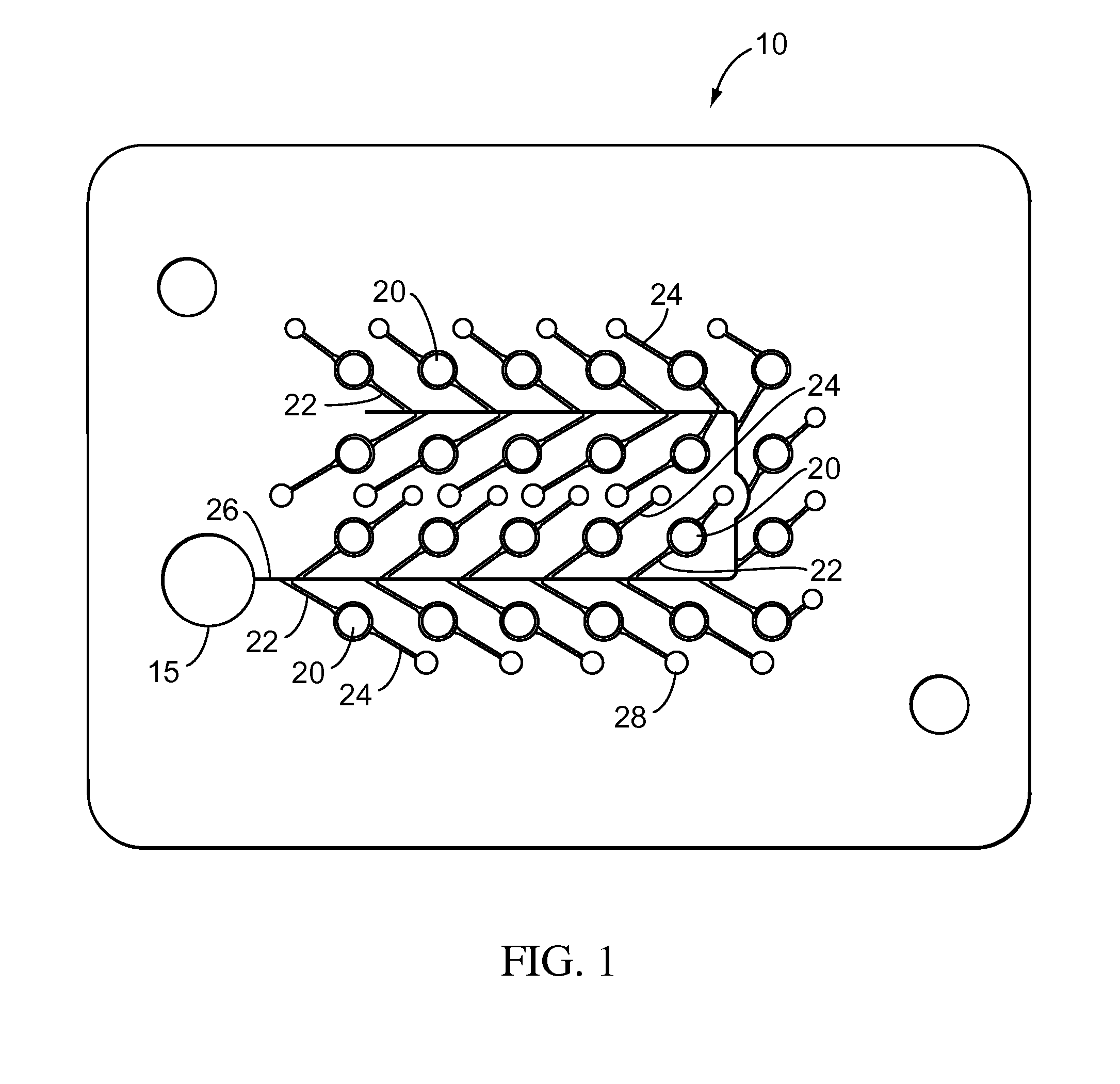
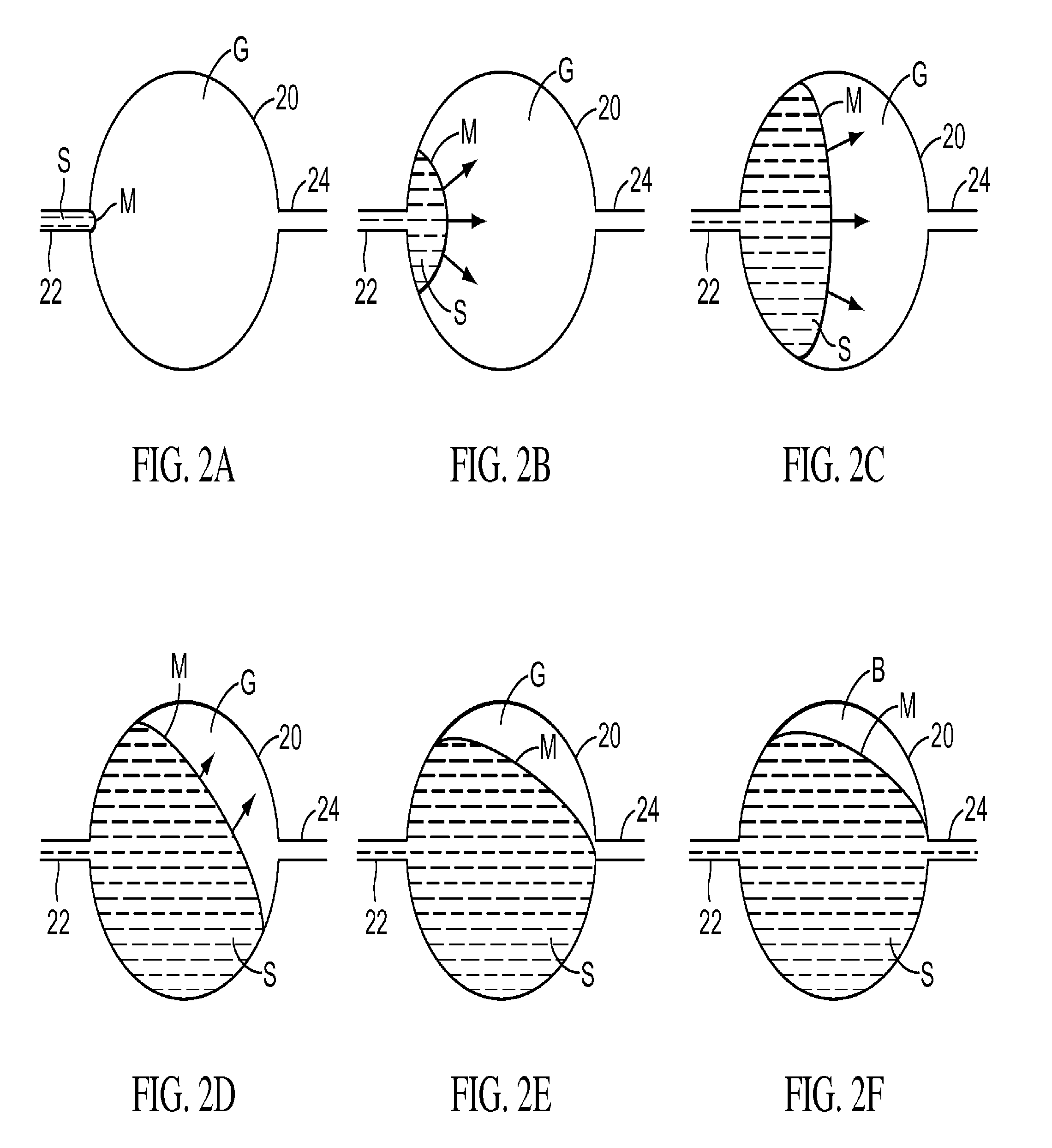
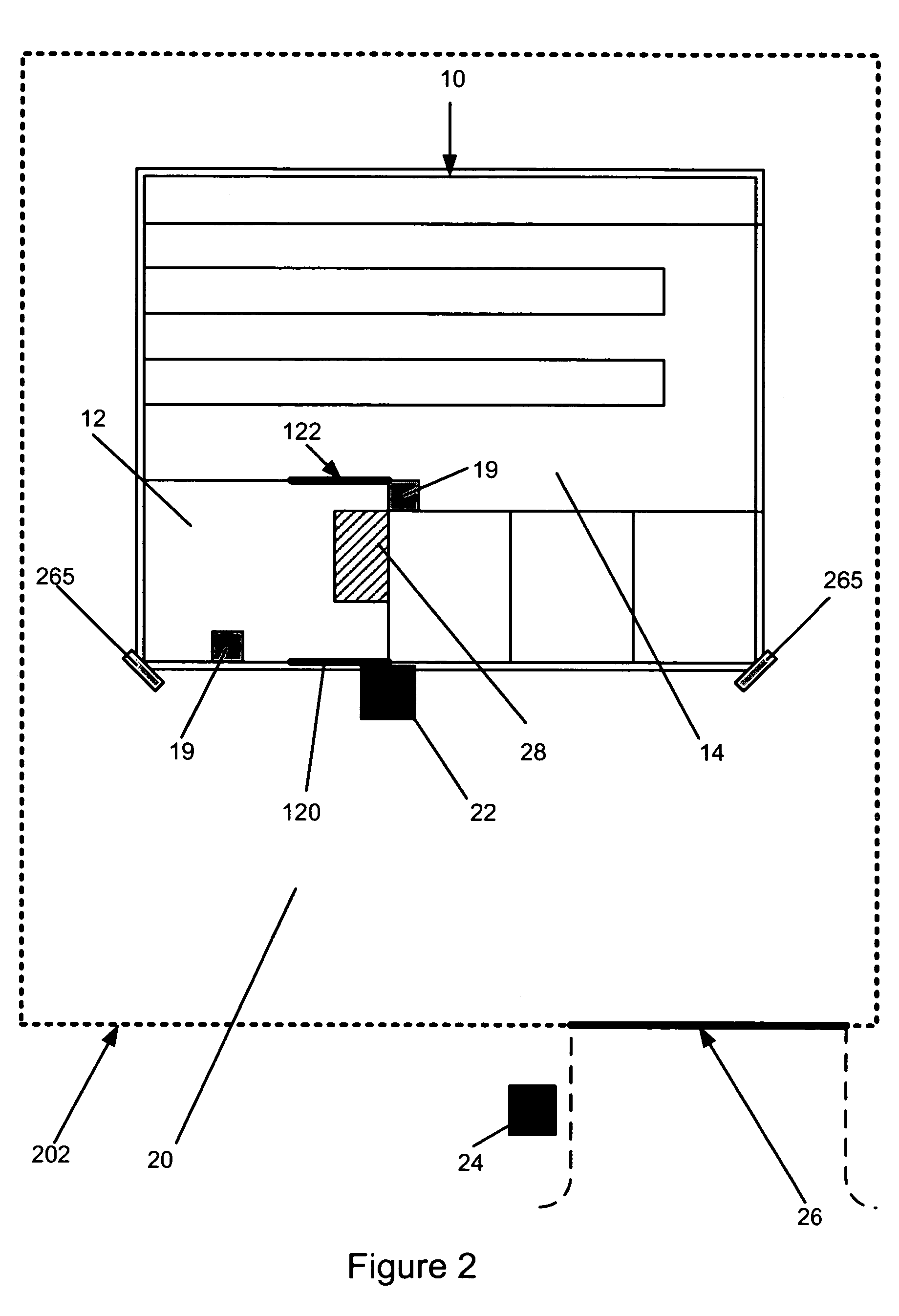
ActiveUS20070249921A1Improve user experienceIncrease probabilityMaterial analysis by electric/magnetic meansDiagnostic recording/measuringElectrical resistance and conductanceContact pad
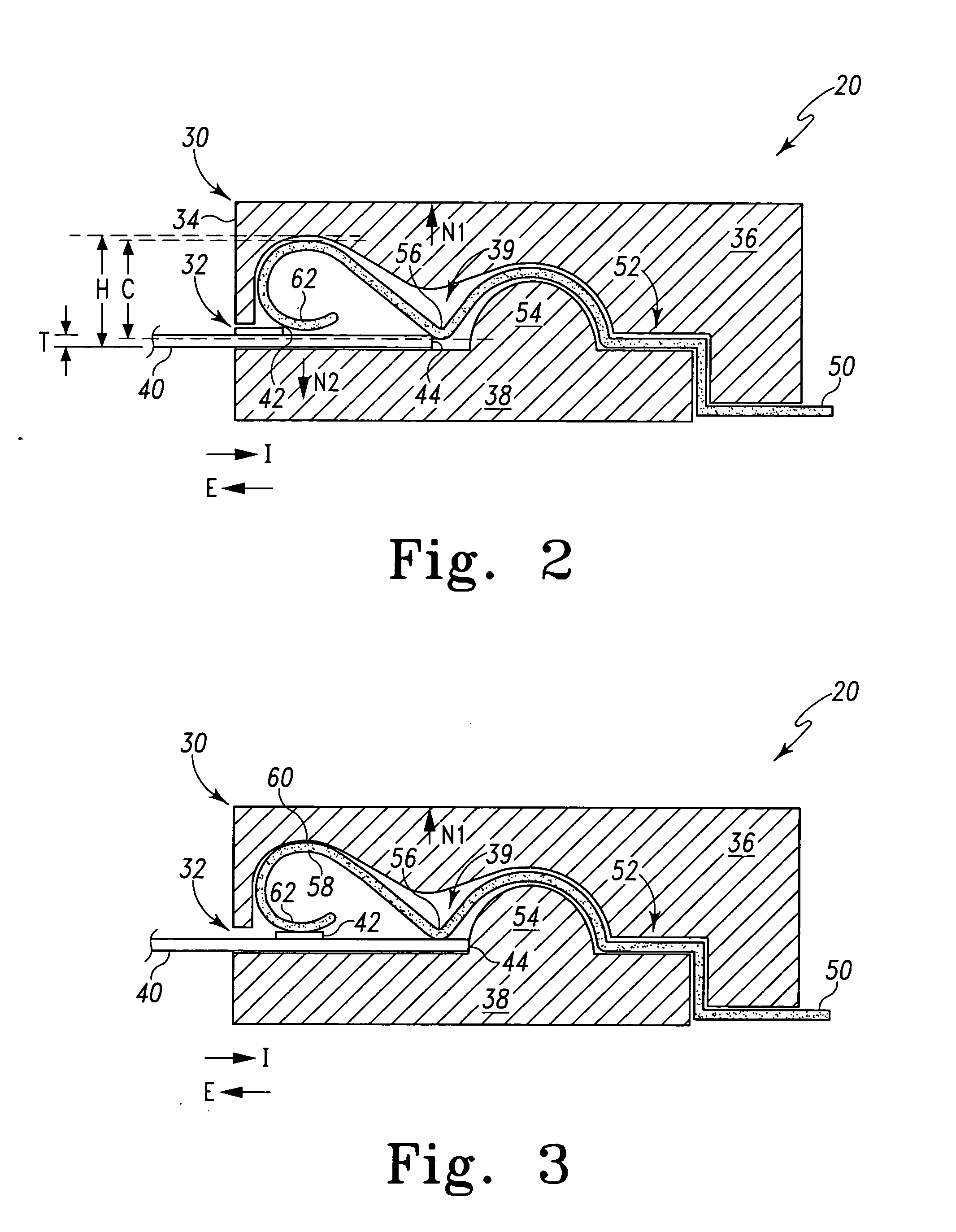
A connector for establishing electrical connection between a testing device and a test strip with a biological fluid thereon includes a contact pad on the test strip, and one or more contact wires in the testing device. When the strip is inserted into the testing device, part of the strip's end engages a contact portion of a contact wire and deflects it in a direction normal to the direction of insertion. In certain embodiments the radius of curvature (in the direction of insertion) of the contact portion is controlled to reduce abrasion of the strip by the wire. In other embodiments the radius of curvature (perpendicular to the direction of insertion) is controlled to reduce the abrasion of the strip by the wire. Sometimes the contact portion and / or contact pad is plated with a sacrificial material to reduce the coefficient of friction. In other embodiments various numbers of contacts receive the end of the strip substantially simultaneously, or are staggered in rows to distribute the resistance presented.
Owner:ROCHE DIABETES CARE INC
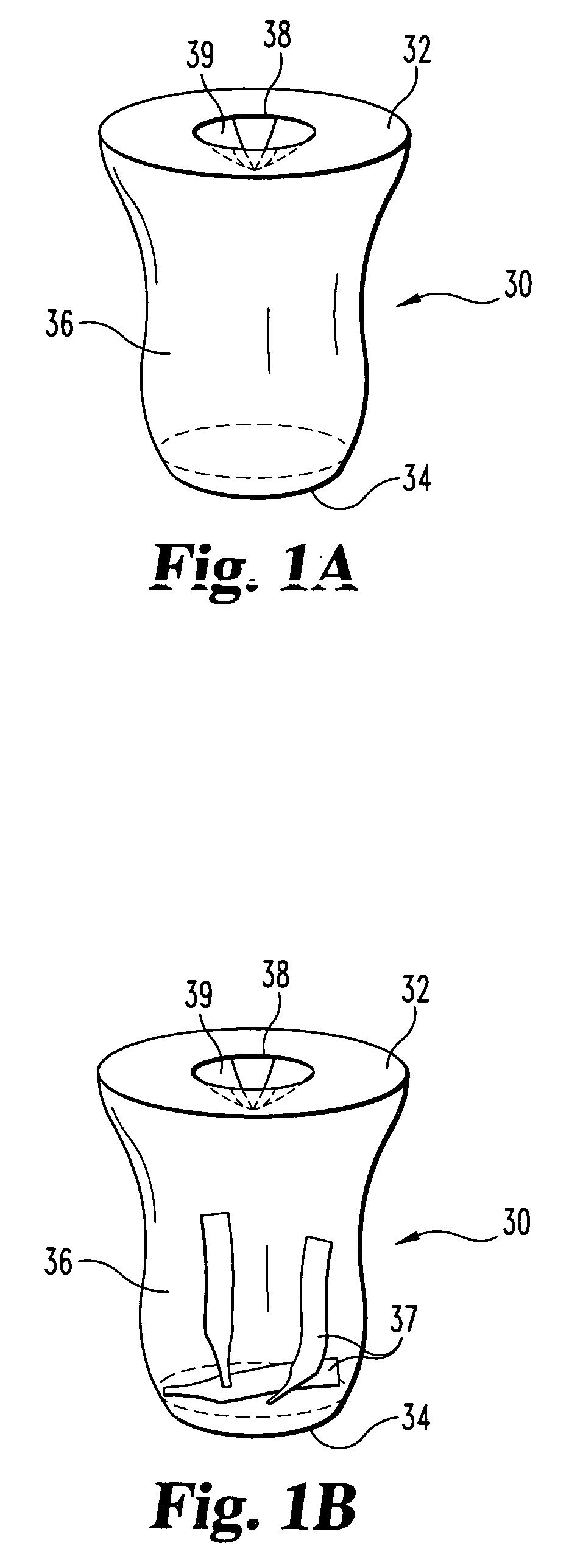
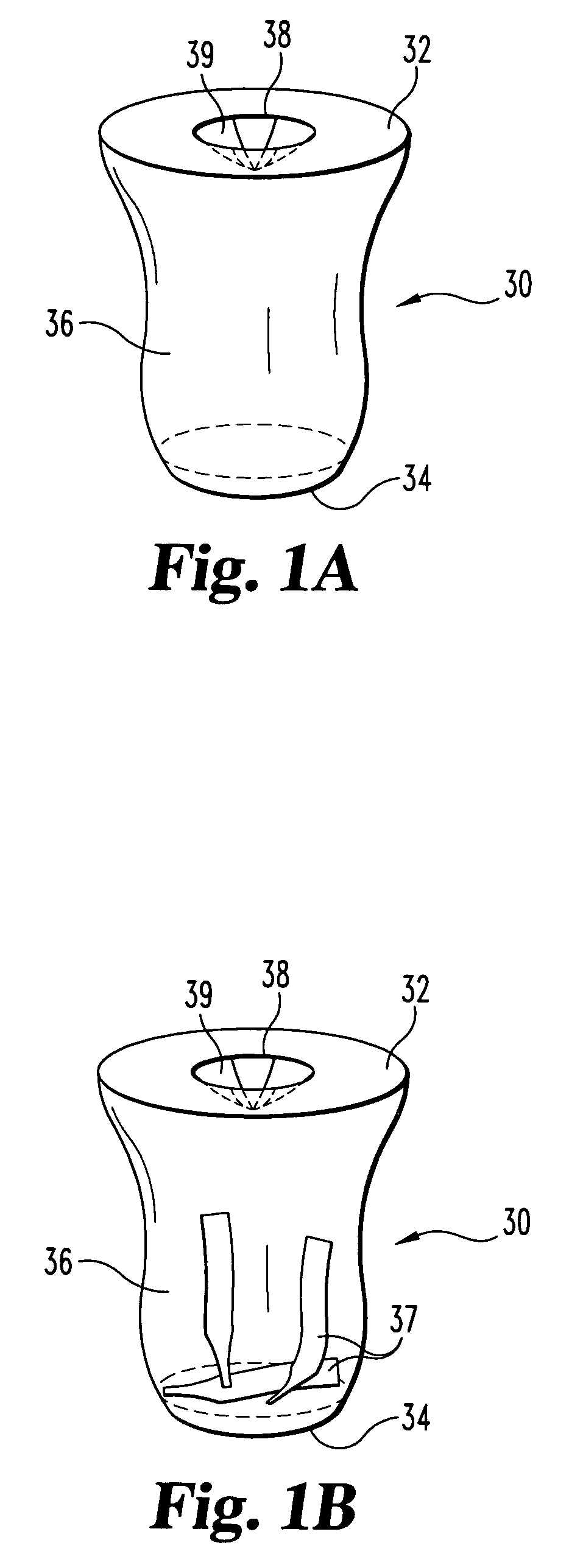
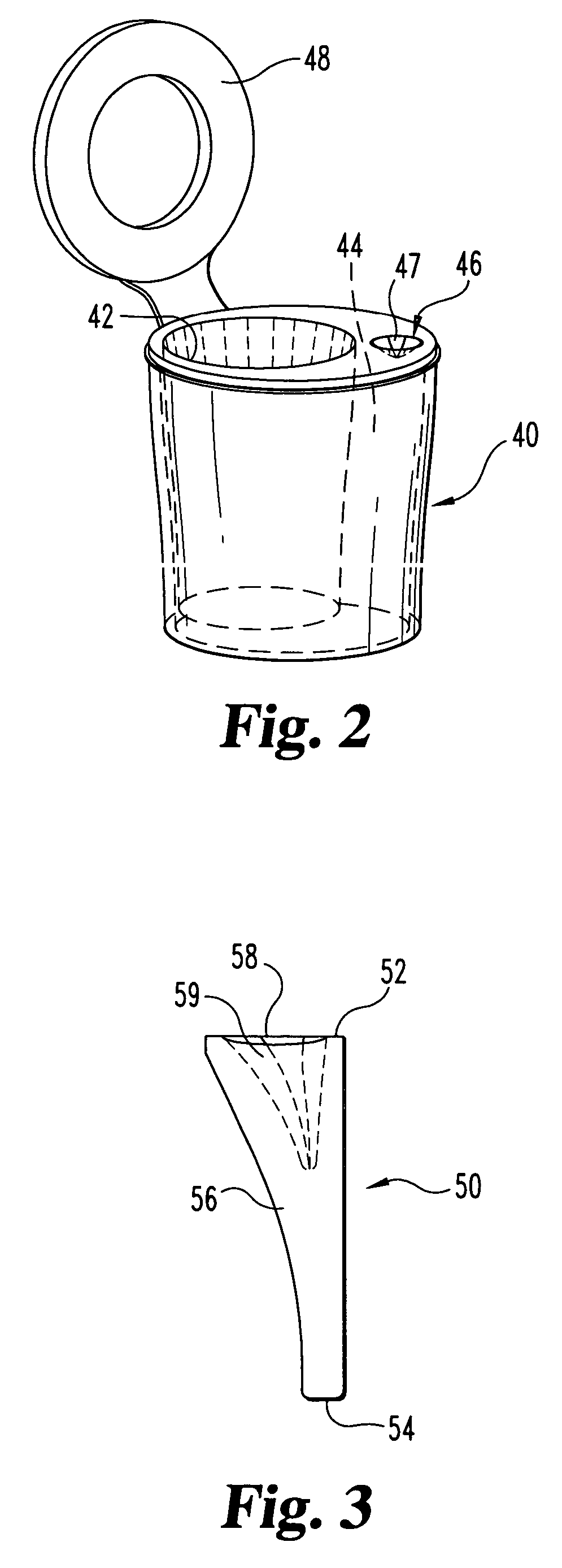
Biocidal blood glucose strip and lancet or sharps disposal device
A disposal receptacle having a biocidal interior surface for storing contaminated biotesting devices therein. The receptacle contains a one-way opening or valve to prevent used biotesting devices and bodily fluid from escaping or being removed from the receptacle; however, the one-way opening allows for insertion of the used biotesting devices therein. The biocidal interior surface and one-way valve in the receptacle form a sanitary method of containing contaminated biotesting devices. This combination allows the user to dispose of the receptacle, and safely and hygienically dispose of any contaminated biotesting devices and bodily fluid therein. The receptacle can be combined with a receptacle for unused biotesting devices, and / or sized to fit with or within a medical device, such as a test meter. In one form, the receptacle can be part of a care kit having a kit container for storing a lancing device, a testing device, and the disposal receptacle.
Owner:ROCHE DIABETES CARE INC
Devices and Methods for Controlling Bubble Formation in Microfluidic Devices
InactiveUS20070280856A1Analysis using chemical indicatorsAnalysis by subjecting material to chemical reactionInlet channelEngineering
A microfluidic device may include a sample distribution network including a plurality of sample chambers configured to be loaded with biological sample for biological testing of the biological sample while in the sample chambers, the biological sample having a meniscus that moves within the sample chambers during loading. The sample distribution network may further include a plurality of inlet channels, each inlet channel being in flow communication with and configured to flow biological sample to a respective sample chamber, and a plurality of outlet channels, each outlet channel being in flow communication and configured to flow biological sample from a respective sample chamber. At least some of the sample chambers may include a physical modification configured to control the movement of the meniscus so as to control bubble formation within the at least some sample chambers. At least some of the sample chambers may include a dried reagent positioned within the at least some sample chambers proximate the inlet channels in flow communication with the at least some sample chambers.
Owner:APPL BIOSYSTEMS INC
Devices and Methods for Positioning Dried Reagent In Microfluidic Devices
InactiveUS20070280857A1Analysis using chemical indicatorsMicrobiological testing/measurementInlet channelEngineering
A microfluidic device may include a sample distribution network including a plurality of sample chambers configured to be loaded with biological sample for biological testing of the biological sample while in the sample chambers, the biological sample having a meniscus that moves within the sample chambers during loading. The sample distribution network may further include a plurality of inlet channels, each inlet channel being in flow communication with and configured to flow biological sample to a respective sample chamber, and a plurality of outlet channels, each outlet channel being in flow communication and configured to flow biological sample from a respective sample chamber. At least some of the sample chambers may include a physical modification configured to control the movement of the meniscus so as to control bubble formation within the at least some sample chambers. At least some of the sample chambers may include a dried reagent positioned within the at least some sample chambers proximate the inlet channels in flow communication with the at least some sample chambers.
Owner:APPL BIOSYSTEMS INC
Method and apparatus to minimize diagnostic and other errors due to transposition of biological specimens among subjects
InactiveUS20120064515A2Burette/pipette supportsMicrobiological testing/measurementBiological bodyReference sample
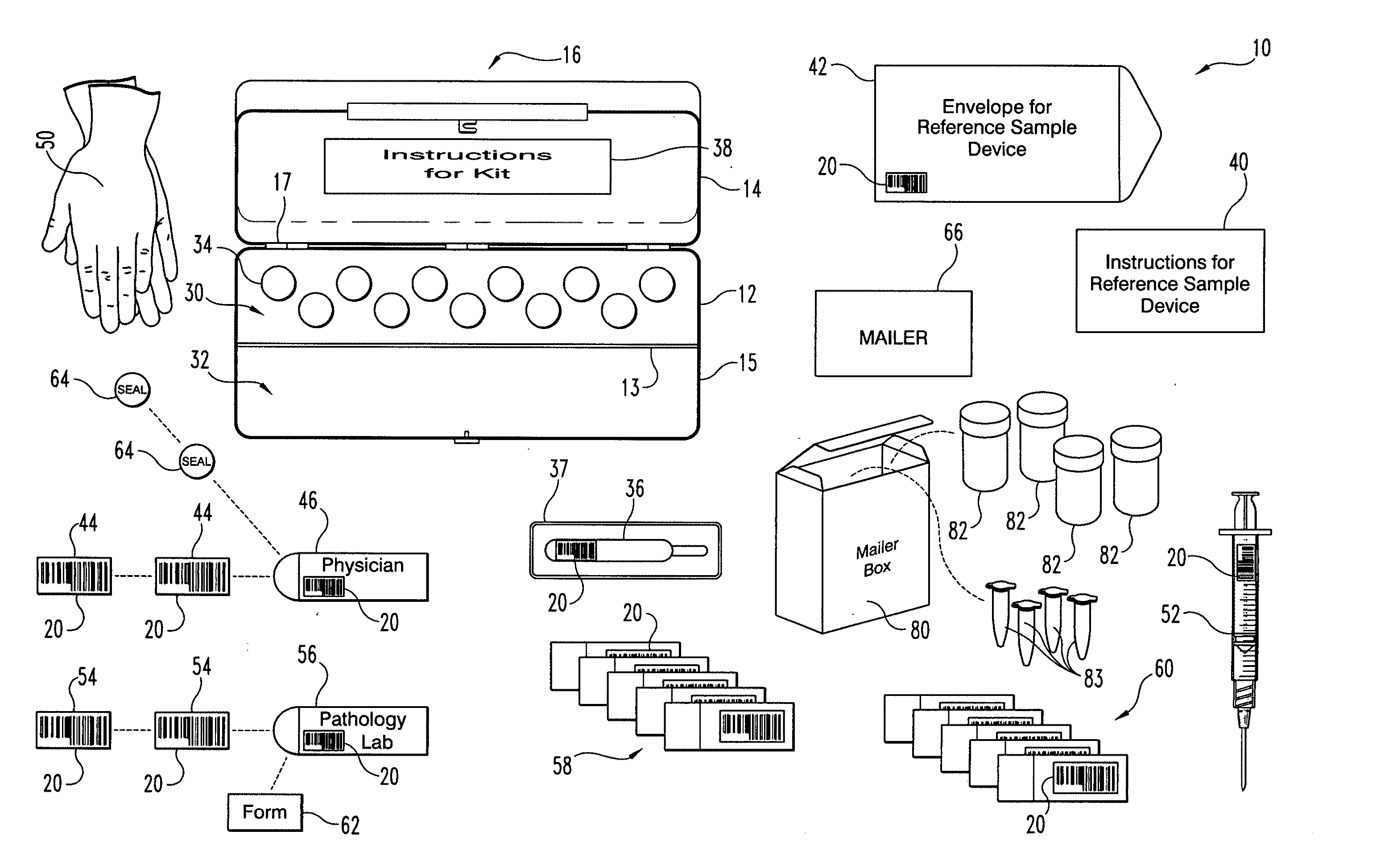
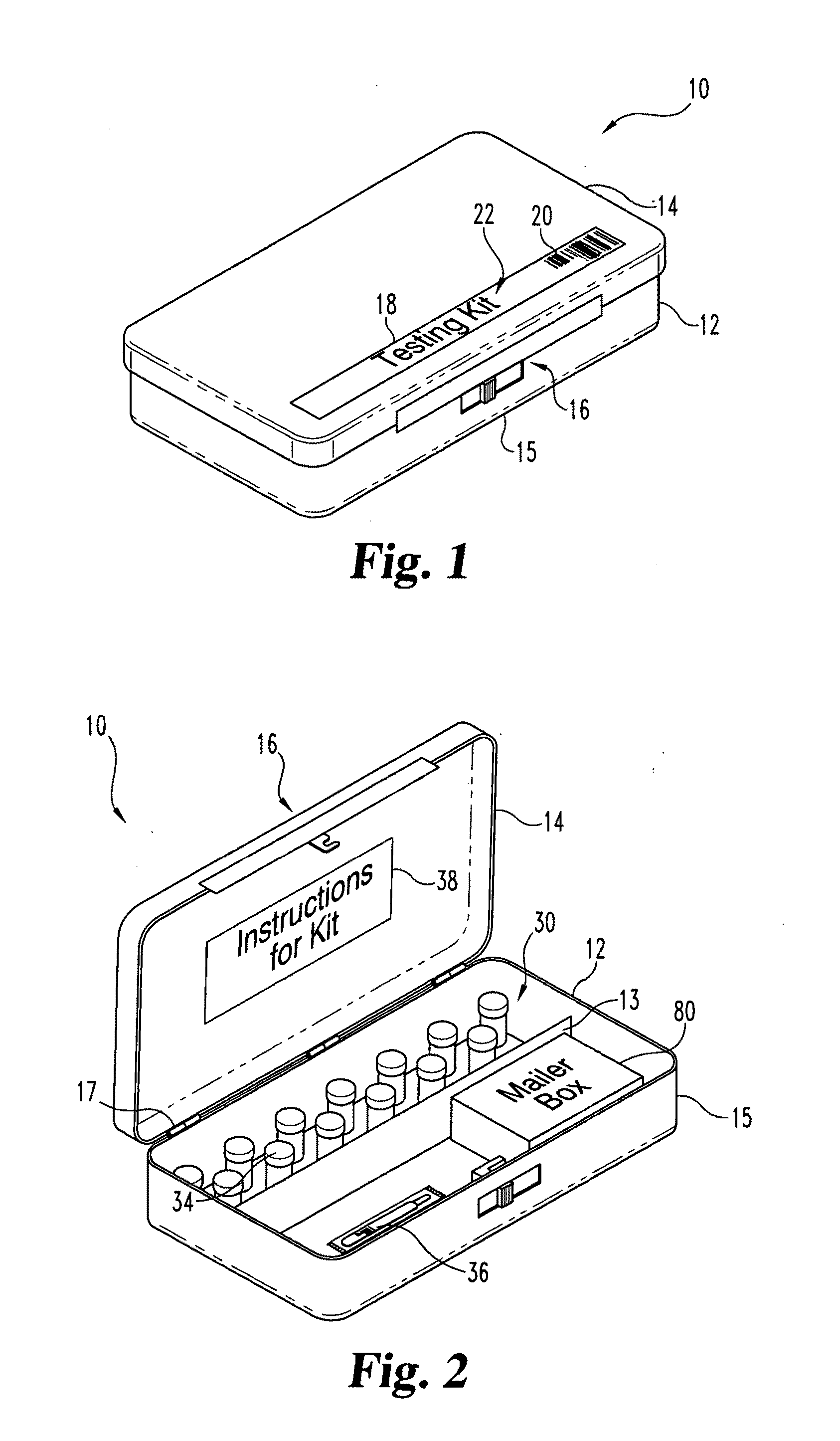
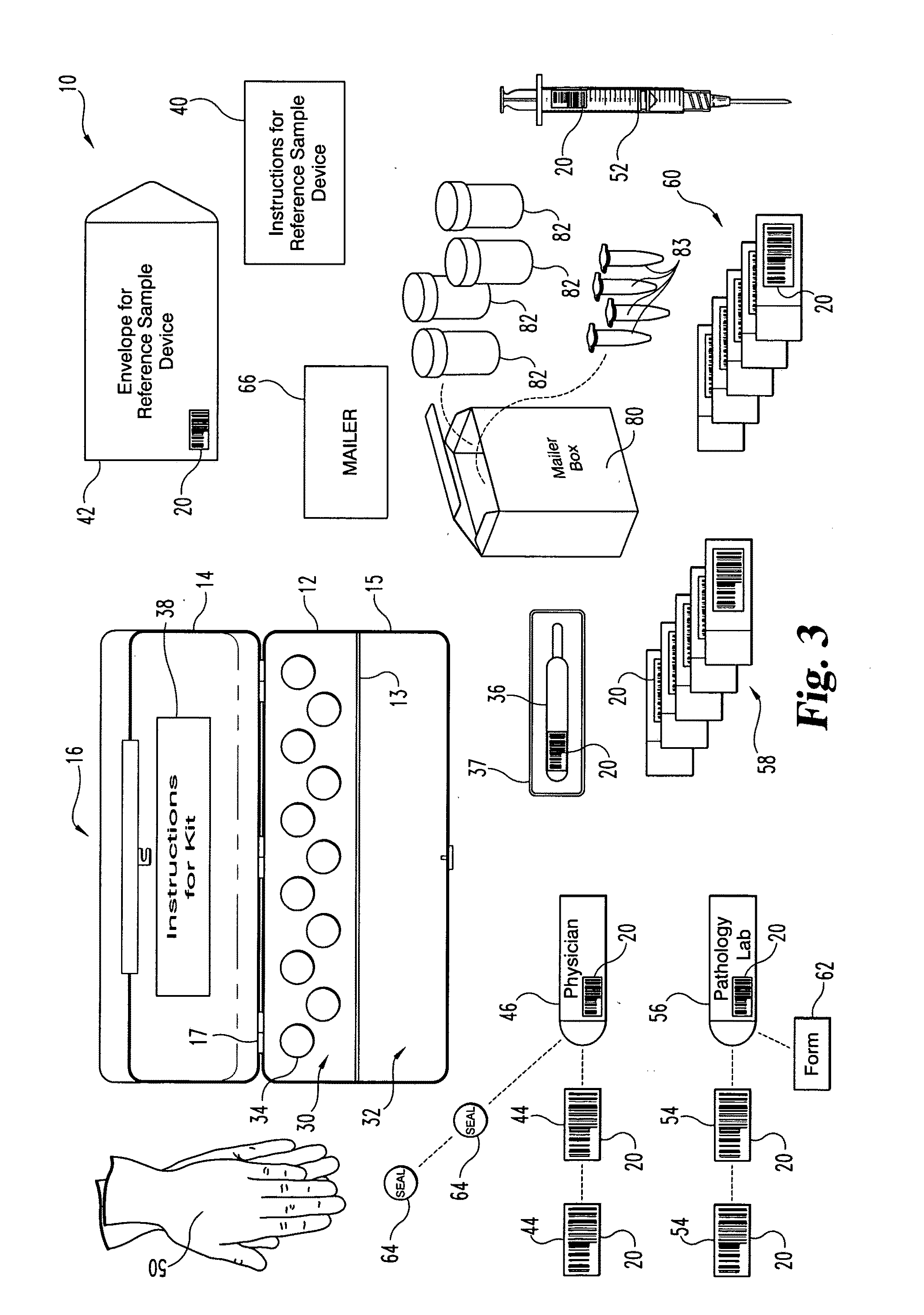
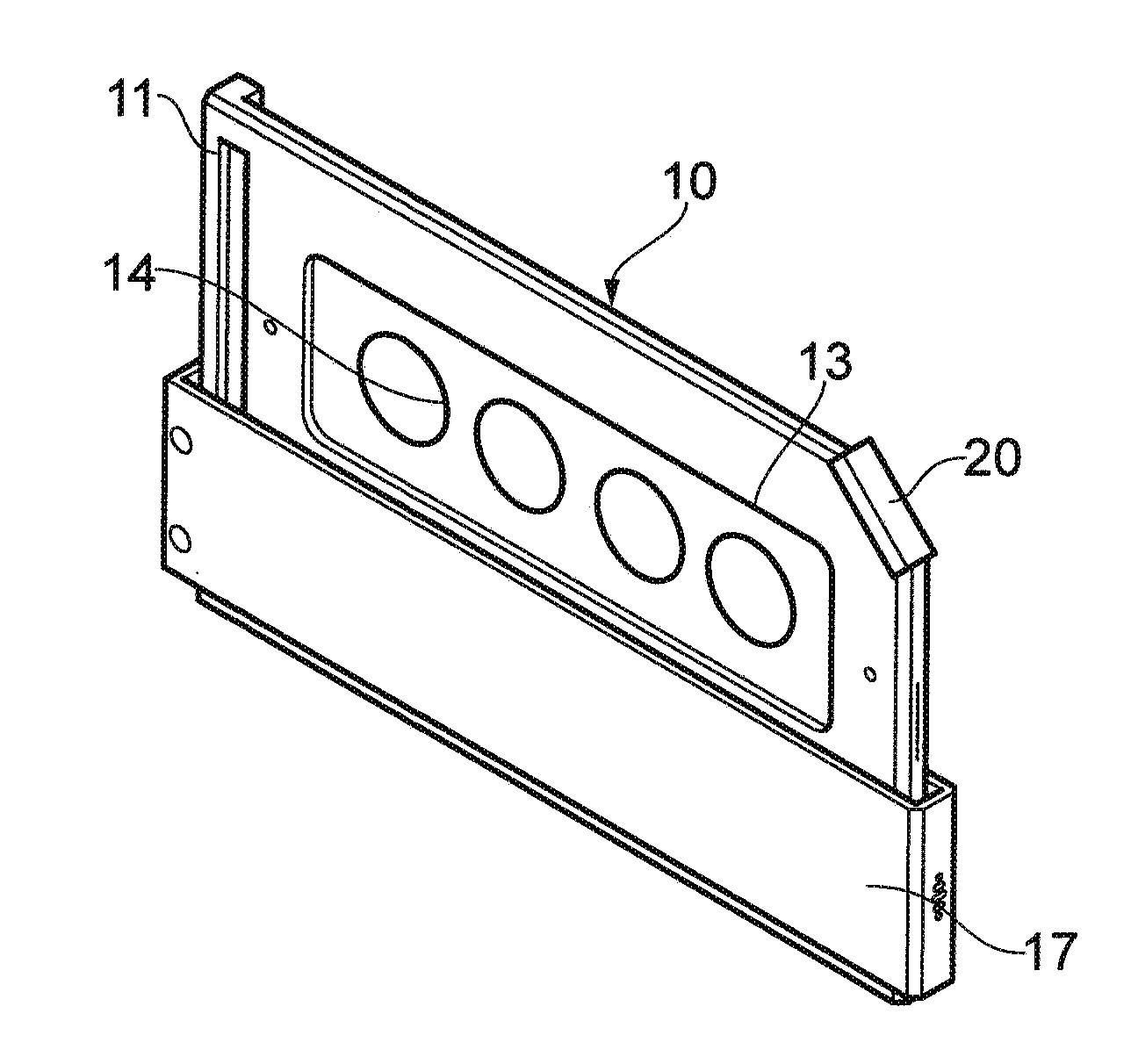
A method and apparatus for minimizing diagnostic errors due to transposition of biological specimens among subjects provides for independent biometric confirmation that a given specimen is from a given donor. In certain embodiments, a biological specimen confirmation kit comprises a portable and openable case housing components of the kit, at least one biological specimen container adapted to receive a biological testing specimen from a donor, and at least one reference sample device adapted to receive a biological reference specimen from the same donor, such that the testing and reference specimens can later be compared for donor match verification by a reference verification entity.
Owner:STRAND DIAGNOSTICS
Sample holder for use in biological testing
InactiveUS20110268630A1Efficient use ofDry fastLaboratory glasswaresSupporting apparatusDesiccantBiological Testing
A holder (2) is provided intended to receive one or more samples of biological fluid for subsequent processing, for example using a robotic punch. The holder has a plastics frame having a windowed portion (3), a substrate (4) for receiving the biological fluid inset within the windowed portion, a cover (7) mounted on the frame slidable between a first position spaced from but overlying the substrate and a second position revealing the substrate and a drying agent (8) positioned on the cover on that side which overlies the substrate in the first position.
Owner:WHATMAN INT
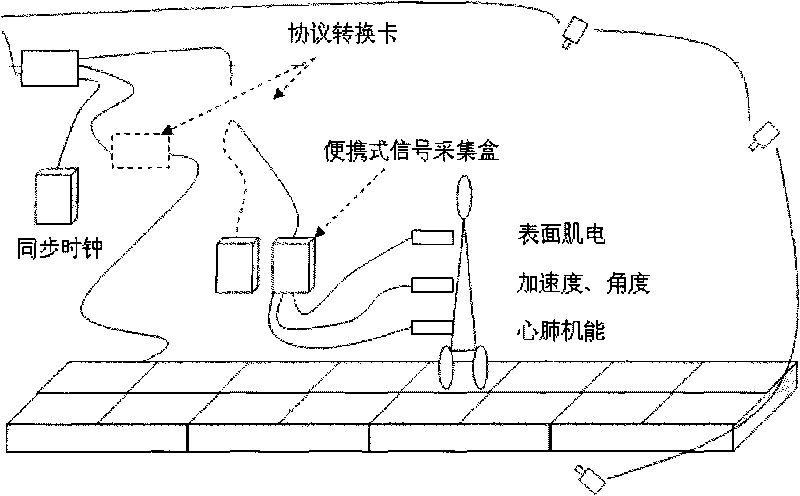
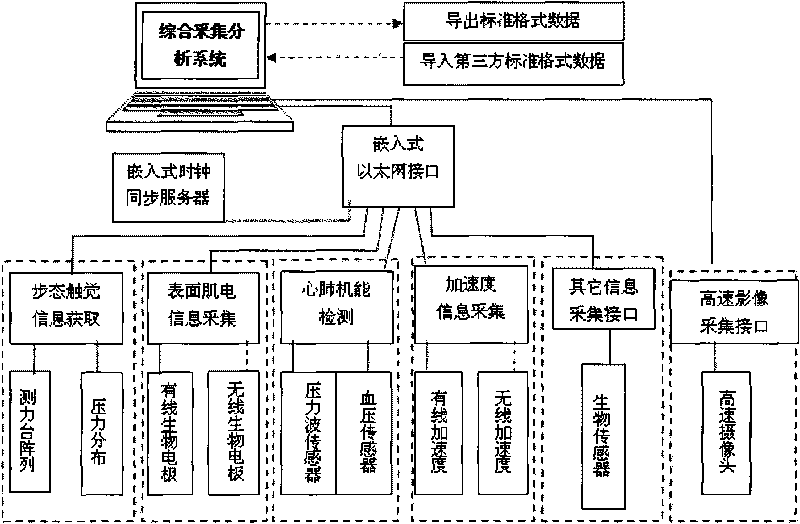
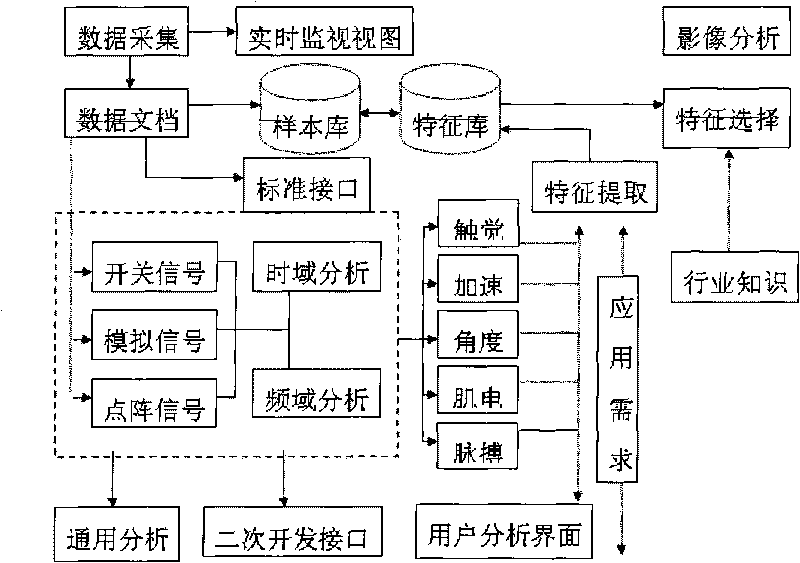
Multiparameter biological information testing platform and testing method
ActiveCN101692977AComprehensive display of mechanical propertiesAchieve acquisitionDiagnostic recording/measuringSensorsSports scienceTest platform
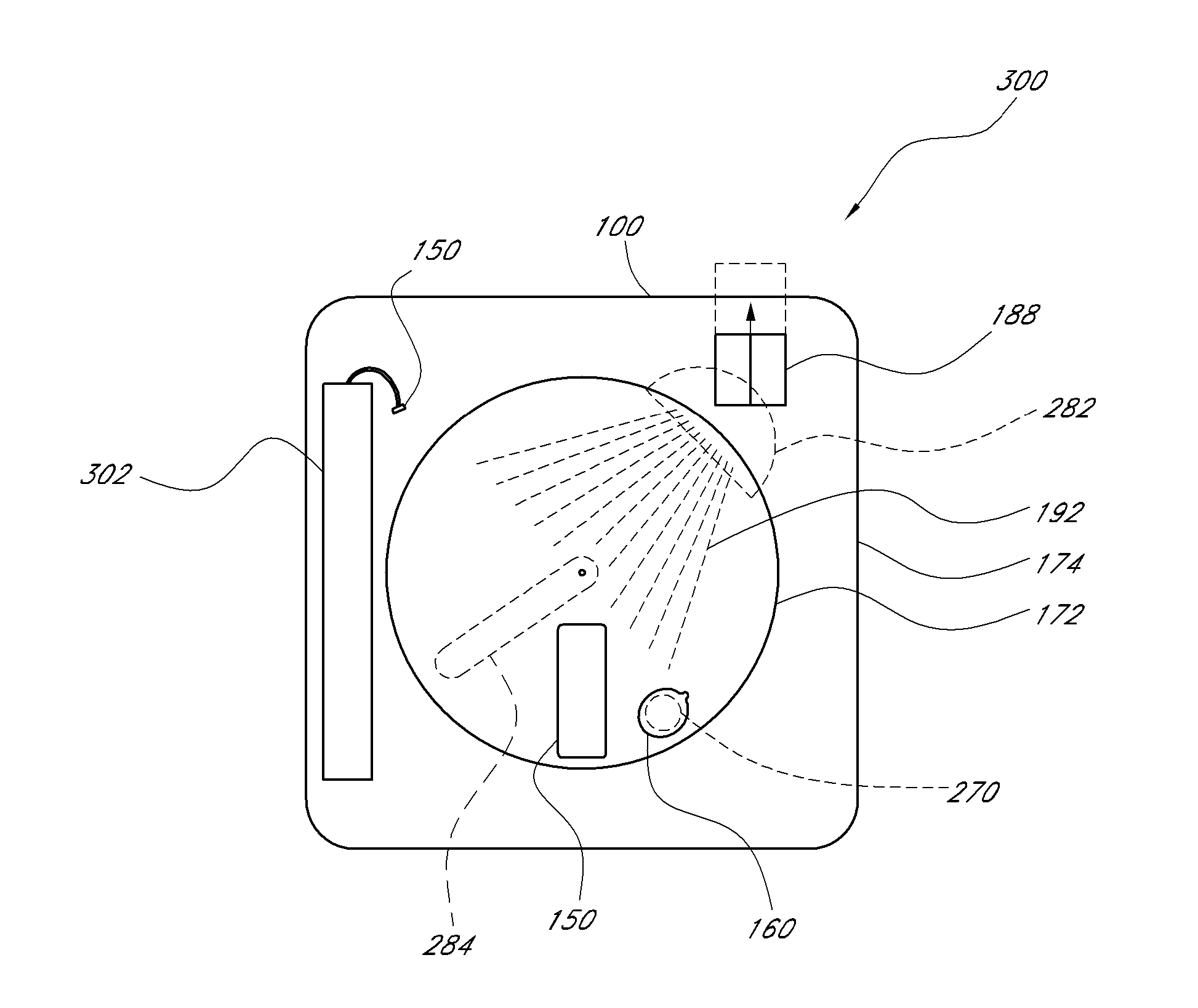
The invention discloses a multiparameter biological information testing platform and a testing method, which adopts a movement human body as a testing object, acquires kinematic parameters, macroscopic action force of a human body and a supporting surface, distribution characteristics of sole pressure, bioelectric information of muscle groups of the human body and the like of all sections synchronously, monitors dynamic change characteristics of cardiopulmonary functions of the human body and provides theory and platform support for relevant studies and applications. The multiparameter biological information testing platform comprises a biological testing channel and integrated analysis software, wherein the testing channel comprises a synchronous clock server, a gait tactile information testing unit, a surface myoelectric acquisition unit, a high-speed image acquisition unit, an acceleration / angle testing unit and a heart rate / pulse testing unit. Under the uniform control of a center computer, all subfunction instruments are interconnected by an Ethernet interface, and realize accurate synchronization of time domains of all parameters by a clock synchronous server to form a comprehensive testing platform; each unit equipment also can be equipped with self-analysis software and a man-machine interface to form an independent instrument. The invention provides a multiparameter biological information acquiring integrated testing device and a testing method for the aspects of gait analysis, sports science research, movement function and health evaluation, basic tests and the like.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
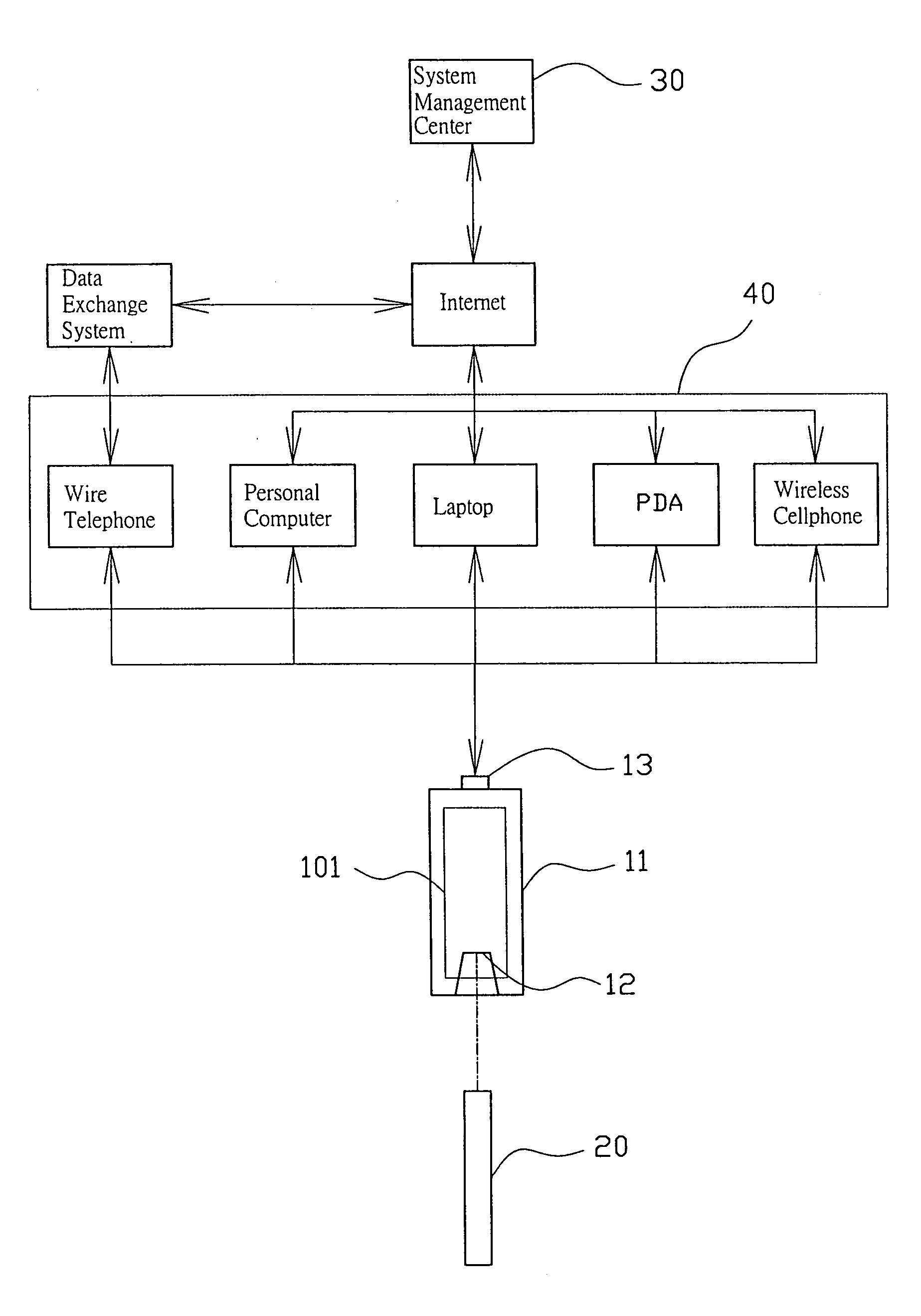
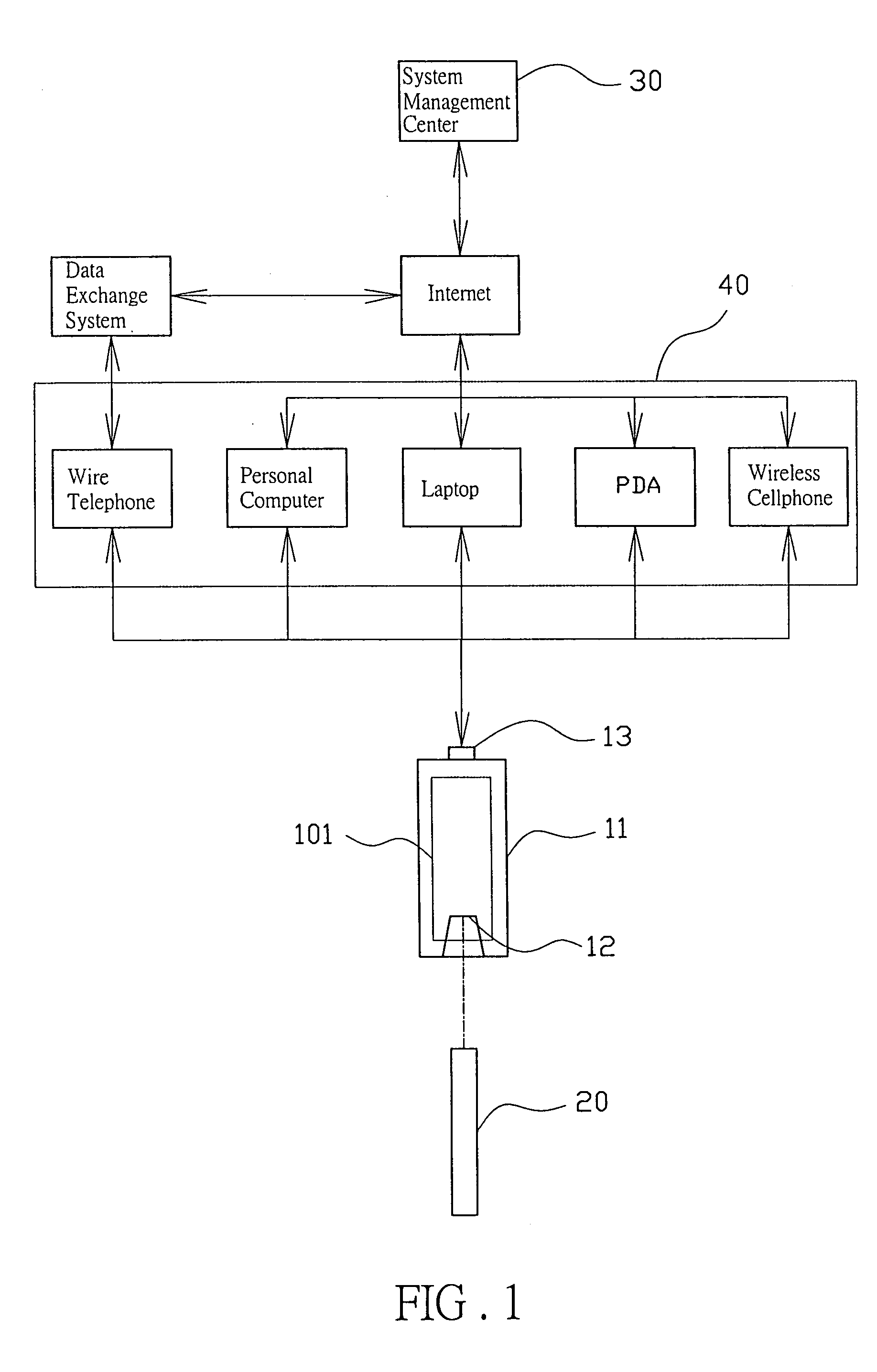
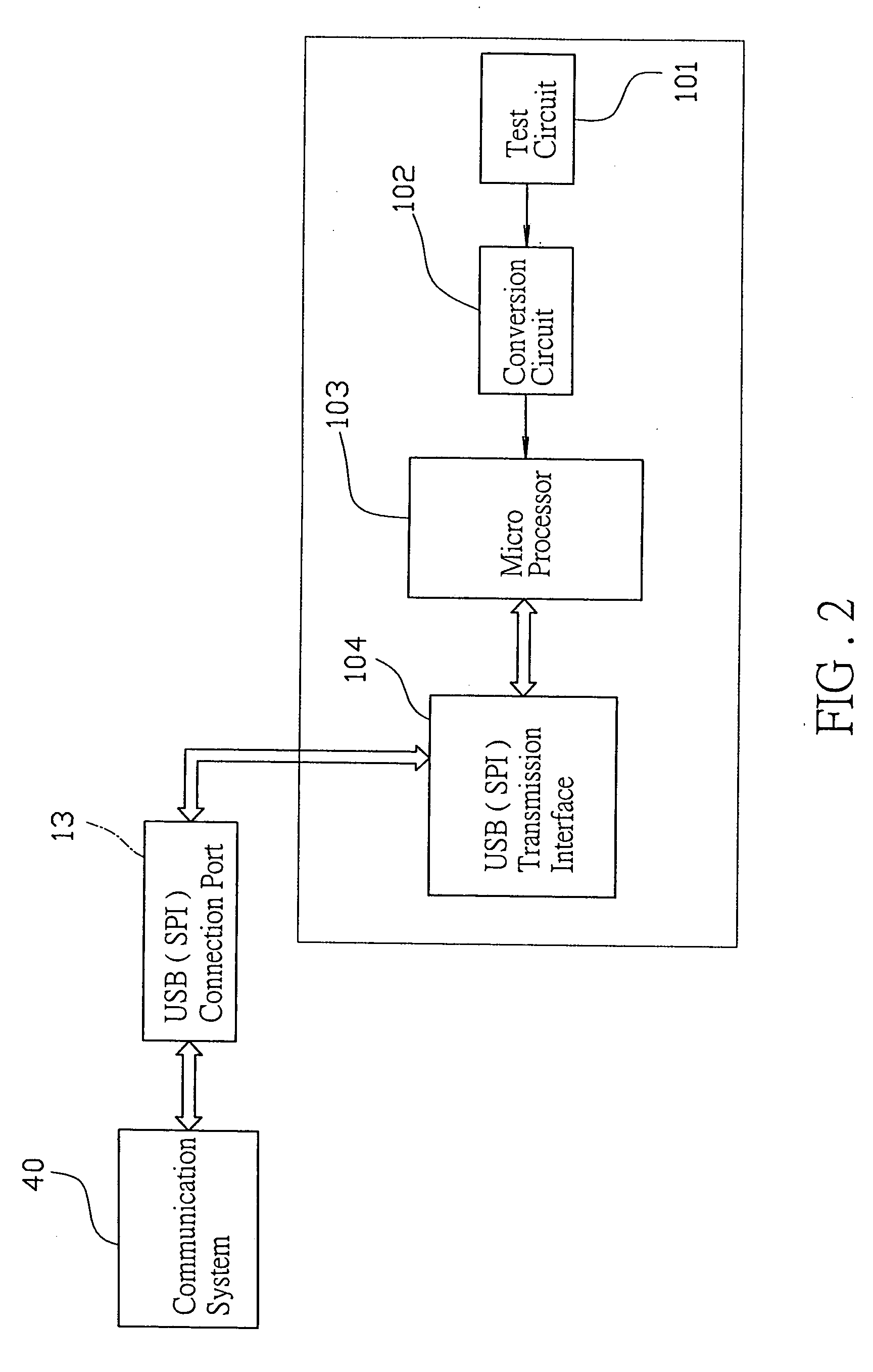
Biological test system
InactiveUS20050096518A1Less tedious for userScope is excellentMicrobiological testing/measurementSensorsThe InternetData profiling
The invention discloses a new biological test system including a biological monitor which can transmit the processed data, analog or digital signals, via a communication port, whether USB, serial or parallel, and a communication media such as telephone, computer or PDA, to the internet server for data analysis and storage. The computer or PDA may download the software for data analysis on line from the web-station without transmitting the data to the internet server.
Owner:CHANG YU HONG
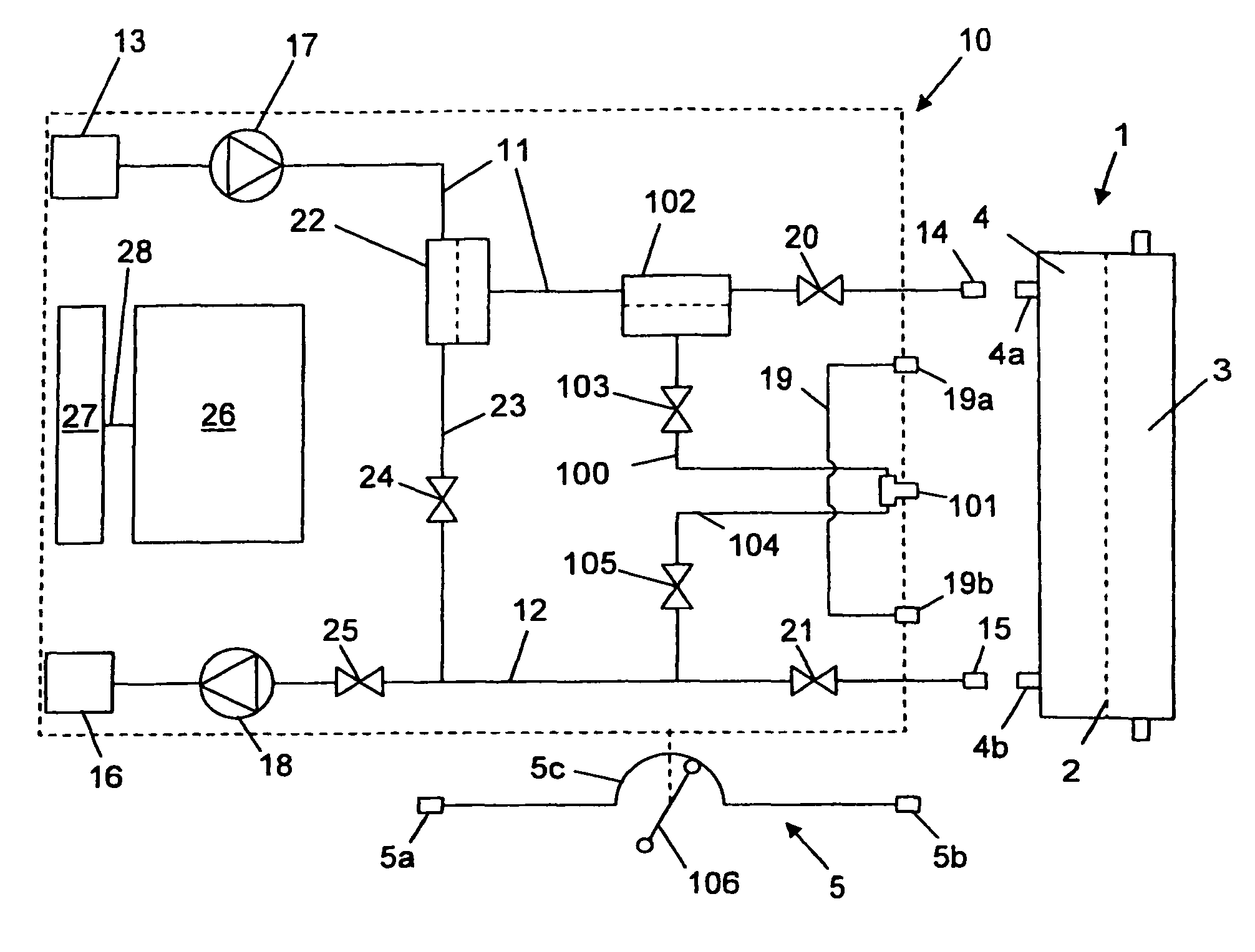
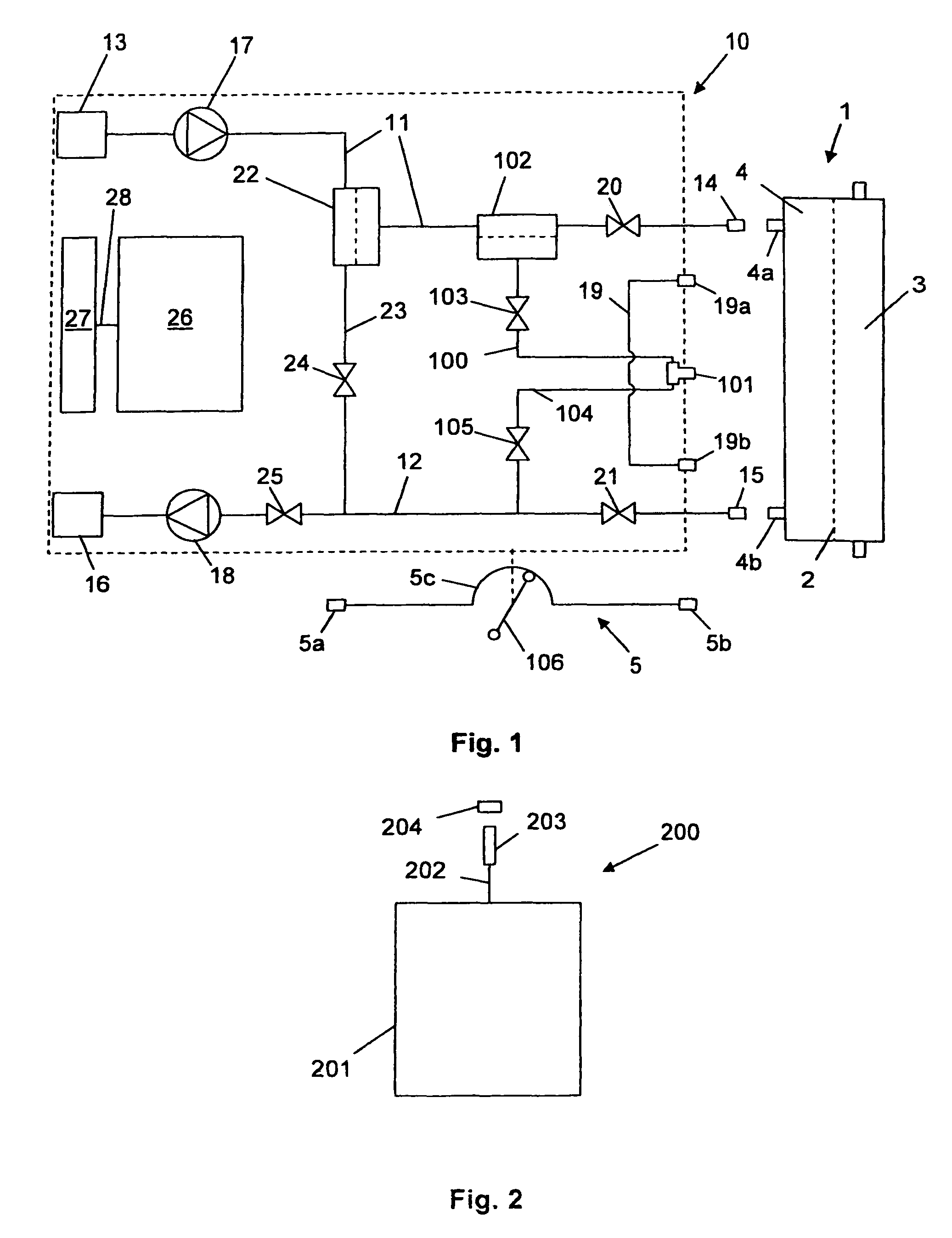
Hemodialysis device, hemodiafiltration device, method for taking a sample in corresponding devices and sampling kit for use in corresponding devices and method
ActiveUS8518258B2Easy to carrySemi-permeable membranesSolvent extractionHaemodialysis machineEngineering
A hemodialysis machine or hemodiafiltration machine is provided that may be used directly for sampling for the connection to a hemodialyzer or for providing replacement fluid, as is required for regular microbiological testing. The machine does not require any additional components or any complex hygiene measures to prevent secondary contamination. By connecting an inventive sterile sampling set, samples can easily be taken. Closing elements and pumping mechanisms are controlled by a control unit as part of a sampling control program.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
Portable biological testing device and method
ActiveUS20080160502A1Avoid biological contaminationBioreactor/fermenter combinationsBiological substance pretreatmentsBiological TestingBiological materials
A device and method for providing portable biological testing capabilities free from biological contamination from an environment outside the device are provided. The device includes a portable housing. The device further includes a volume surrounded by the housing and sealed against passage of biological materials between the volume and the environment outside the device. The device further includes a culture medium within the volume. The device further includes one or more ports configured to provide access to the volume while avoiding biological contamination of the volume. The device further includes a valve in fluidic communication with the volume and the environment. The valve has an open state in which the valve allows gas to flow from within the volume to the environment outside the device and a closed state in which the valve inhibits gas from flowing between the volume and the environment. The valve switches from the closed state to the open state in response to a pressure within the volume larger than a pressure of the environment outside the device.
Owner:BARNES ALLEN C +1
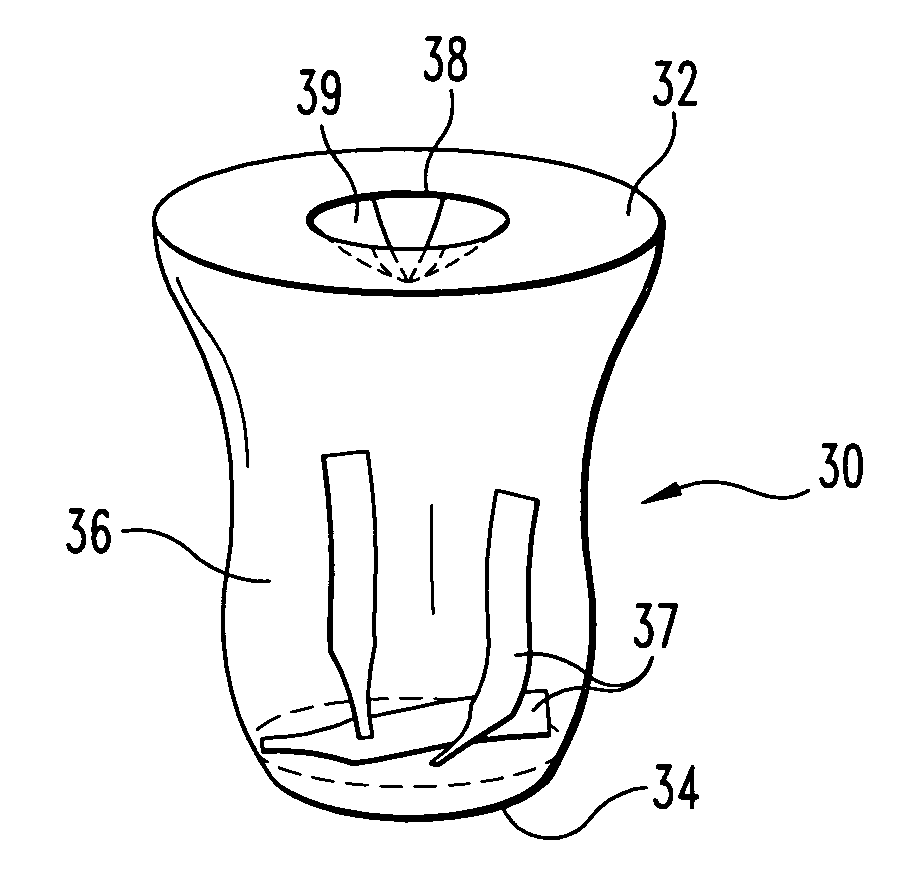
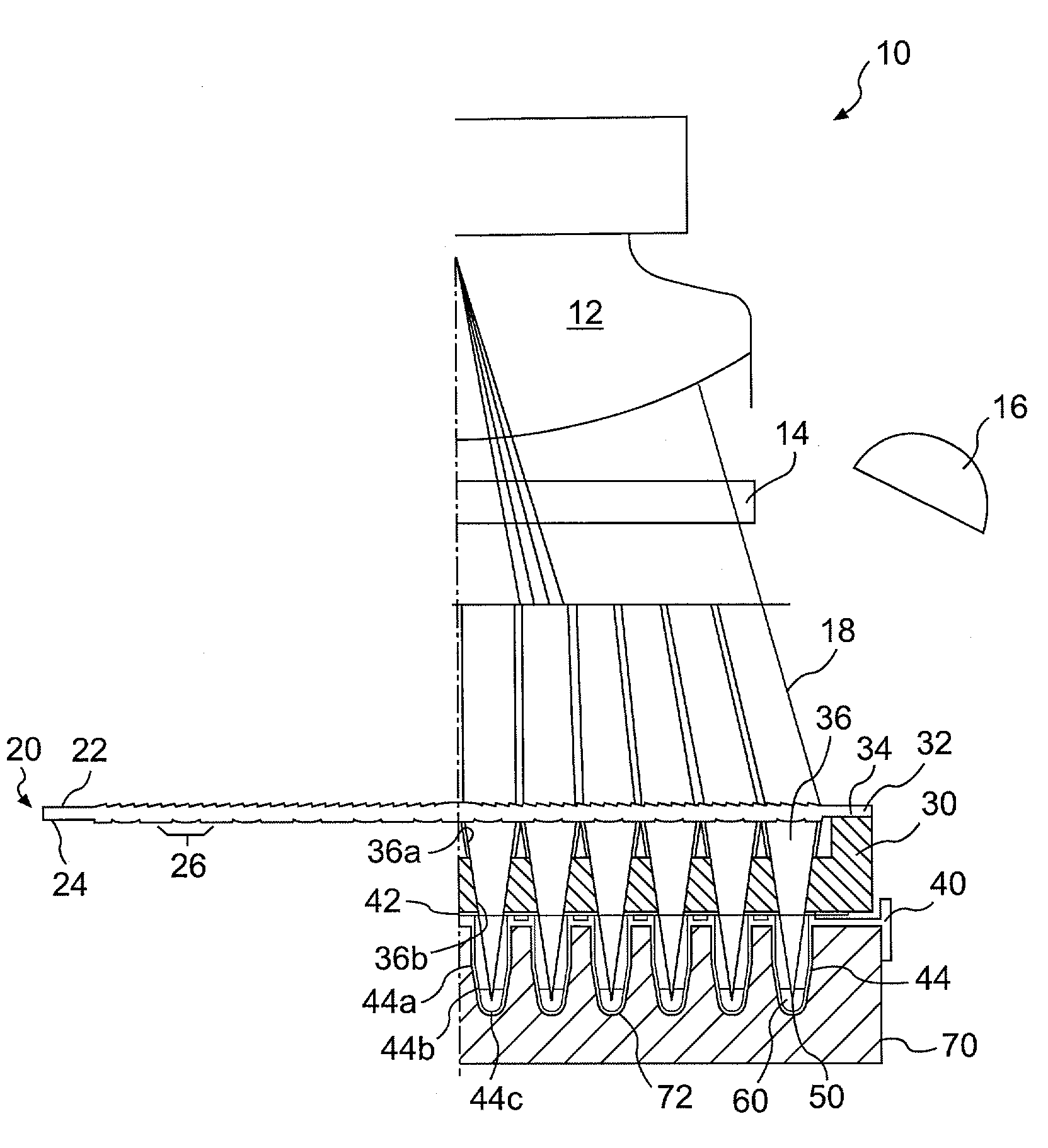
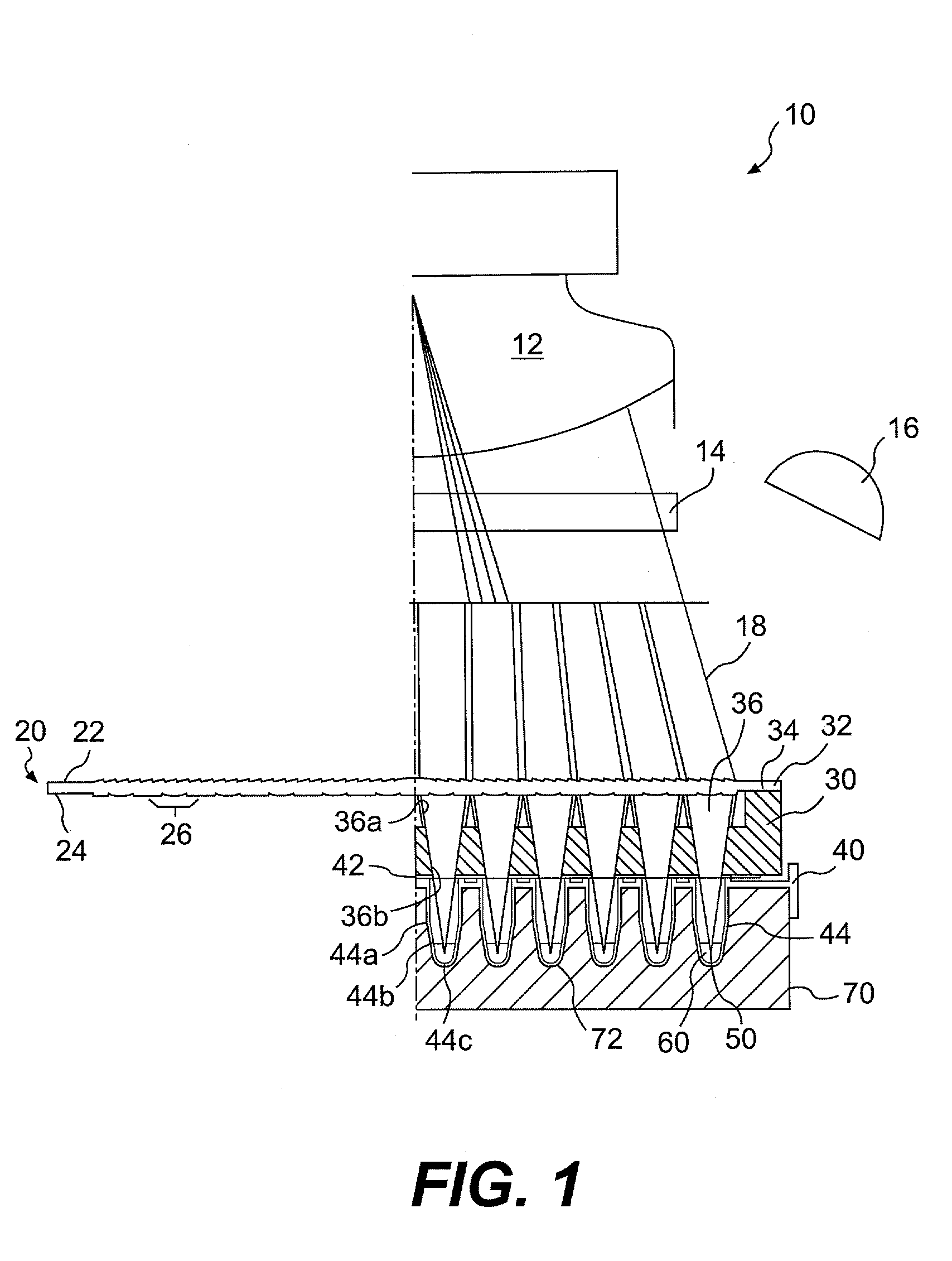
Lens assembly for biological testing
InactiveUS20030215938A1Bioreactor/fermenter combinationsHeating or cooling apparatusCamera lensBiological Testing
The invention relates to an optical detection system for a thermal cycling device including at least one light source, a light detection device for detecting light received from a plurality of biological samples, and a lens having first and second surfaces formed on the lens, the second surface substantially opposed to the first surface. The first surface may be configured to collimate light and the second surface may be configured to direct light into each of the plurality of biological samples.
Owner:APPL BIOSYSTEMS INC
Method and system for production and collection of lavage induced stool (LIS) for chemical and biologic tests of cells
InactiveUS6447763B1Improve practicalityImprove cost effectivenessDigestive systemSurgeryDiseaseFeces
Beverages are provided and administered for producing LIS samples containing cells exfoliated from throughout the gut in sufficient numbers and free of interfering substances such as formed fecal particles for chemical assays and biologic assays for nucleic acid sequence information, and medical diagnosis. A kit is also provided for use by patients without assistance to produce a LIS sample suitable for analysis. A collection kit employs a sequence of the beverages and other ingested substances to produce preserved cells for medical diagnosis, allowing cytologic analysis of the LIS for diagnosis of foregut and hindgut disease. A preliminary cathartic lavage is used to cleanse a patient's digestive tract; at least one stool induced by the preliminary cathartic lavage is collected; and a final cathartic lavage is used to exfoliate and preserve cells from a patient's digestive tract. Time release capsules containing a cathartic medicament can also be used after completing preliminary lavage administration. The kit also allows provides apparatus for collection, sealing, and packing of the collected LIS specimen for analysis.
Owner:GORDON IAN L
Stopping bleeding spraying adhesive, preparing method and use thereof
A spray-type staltic adhesive is prepared from the alpha-n-octyl cynoacrylate (1-50 wt.%) and the alpha-n-butyl cynoacrylate (1-80 wt.%) through proportioning, sequentially loading them in a container, stirring for 30-80 min, filtering by 0.45-micron medical film, and biological testing to be negative. Its advantages are high polymerizing speed, adhesion and diffusivity, less intradermal reaction, and high safety. It can be used in human body.
Owner:FUALIE SCI & TECH DEVING BEIJING
Lens assembly for biological testing
InactiveUS6982166B2Bioreactor/fermenter combinationsHeating or cooling apparatusCamera lensBiological Testing
The invention relates to an optical detection system for a thermal cycling device including at least one light source, a light detection device for detecting light received from a plurality of biological samples, and a lens having first and second surfaces formed on the lens, the second surface substantially opposed to the first surface. The first surface may be configured to collimate light and the second surface may be configured to direct light into each of the plurality of biological samples.
Owner:APPL BIOSYSTEMS INC
Devices And Method For Positioning Dried Reagent In Microfluidic Devices
InactiveUS20140141438A1Bioreactor/fermenter combinationsBiological substance pretreatmentsInlet channelEngineering
Owner:APPL BIOSYSTEMS INC
Business method of implementing an automated vault machine
ActiveUS7497376B2Remove restrictionsOptimize locationComplete banking machinesFinanceBiological TestingVisual identification
Owner:LANDWIRTH DONALD M
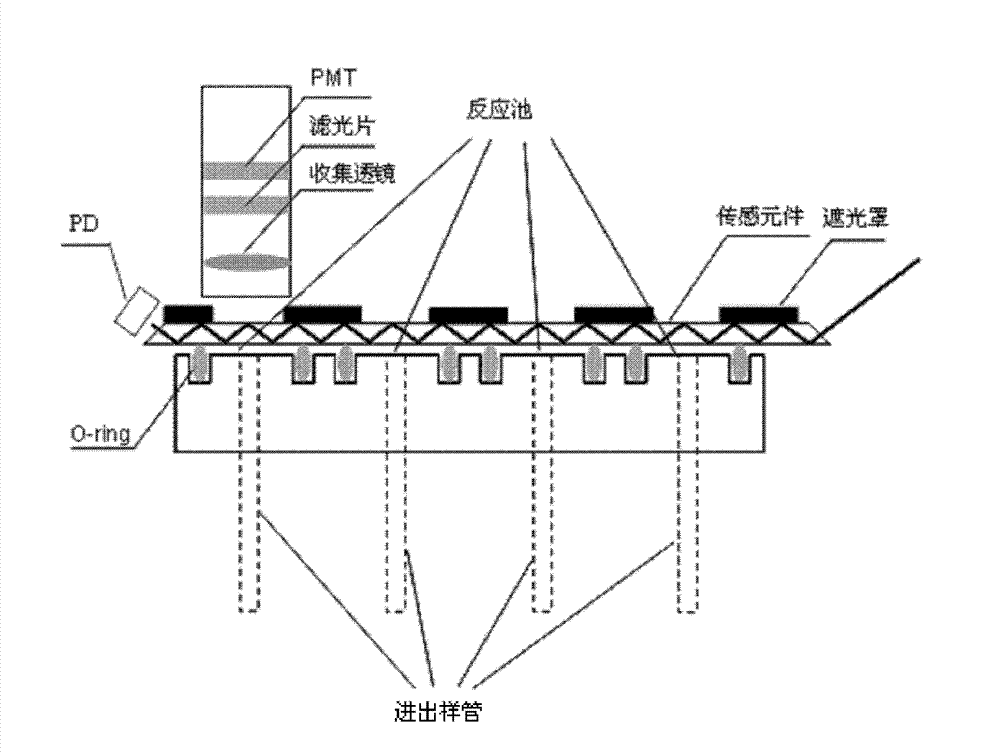
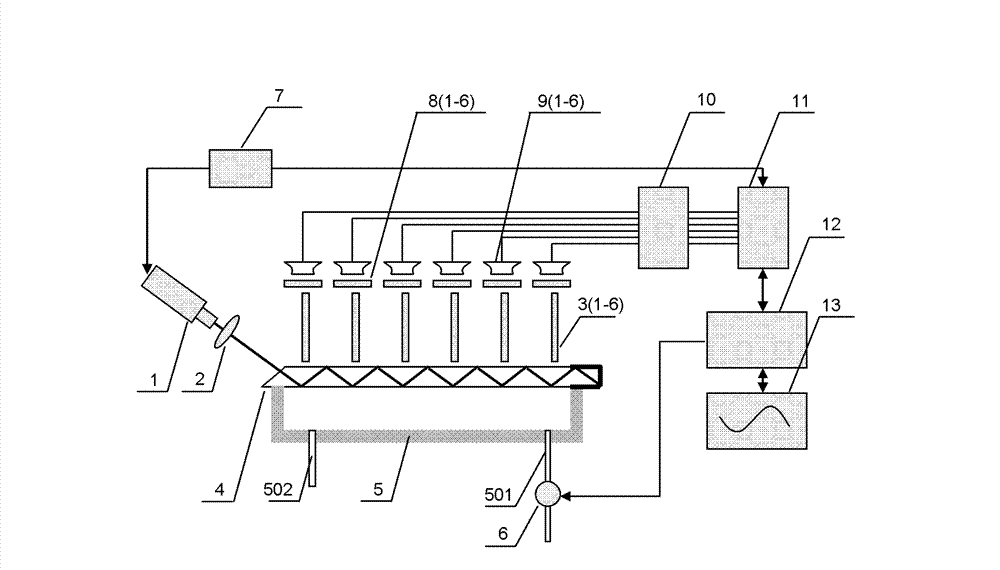
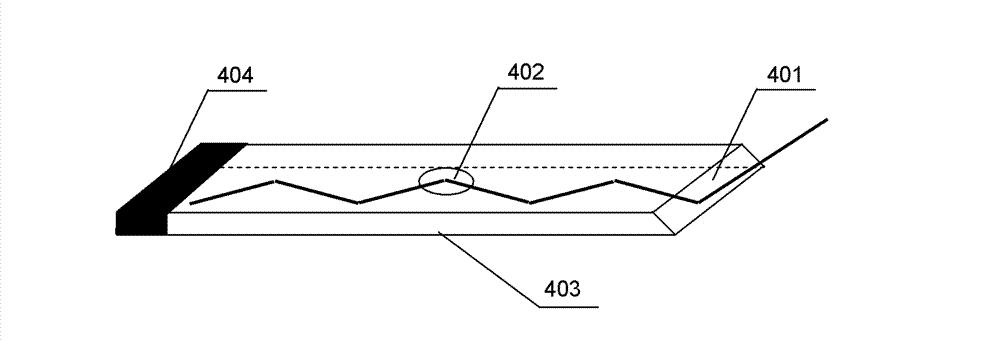
Multi-channel planar waveguide evanescent wave biosensor
InactiveCN103245641ASmall error in parallelismTotal reflection points have the same conditionsFluorescence/phosphorescenceFiberFluorescence
The invention provides a multi-channel planar waveguide evanescent wave biosensor, belongs to the technical field of biological testing, and particularly relates to fluorescent dye-labeled biological substance luminescence excited by laser, thereby achieving the biological detection technology. The biosensor is characterized in that: in the process of laser optical waveguide in a flat glass medium, a plurality of total reflection points are formed on the interface of two-phase medium, fluorescent labeled biological molecules in the point spatial range are excited by evanescent waves generated at the total reflection points to generate fluorescence, the fluorescence is collected by multimode fibers, and fluorescence signals are detected by a lock-in amplifier. As the laser in planar waveguide process can form a plurality of total reflection points, each total reflection point is marked with different biological molecules and the fluorescence signal is detected separately, simultaneous determination of multi-index in a sample can be achieved. The biosensor has the characteristics of simple waveguide structure, high fluorescence collection efficiency, small background noise interference, enabling simultaneous determination of multi-index and the like.
Owner:TSINGHUA UNIV +1
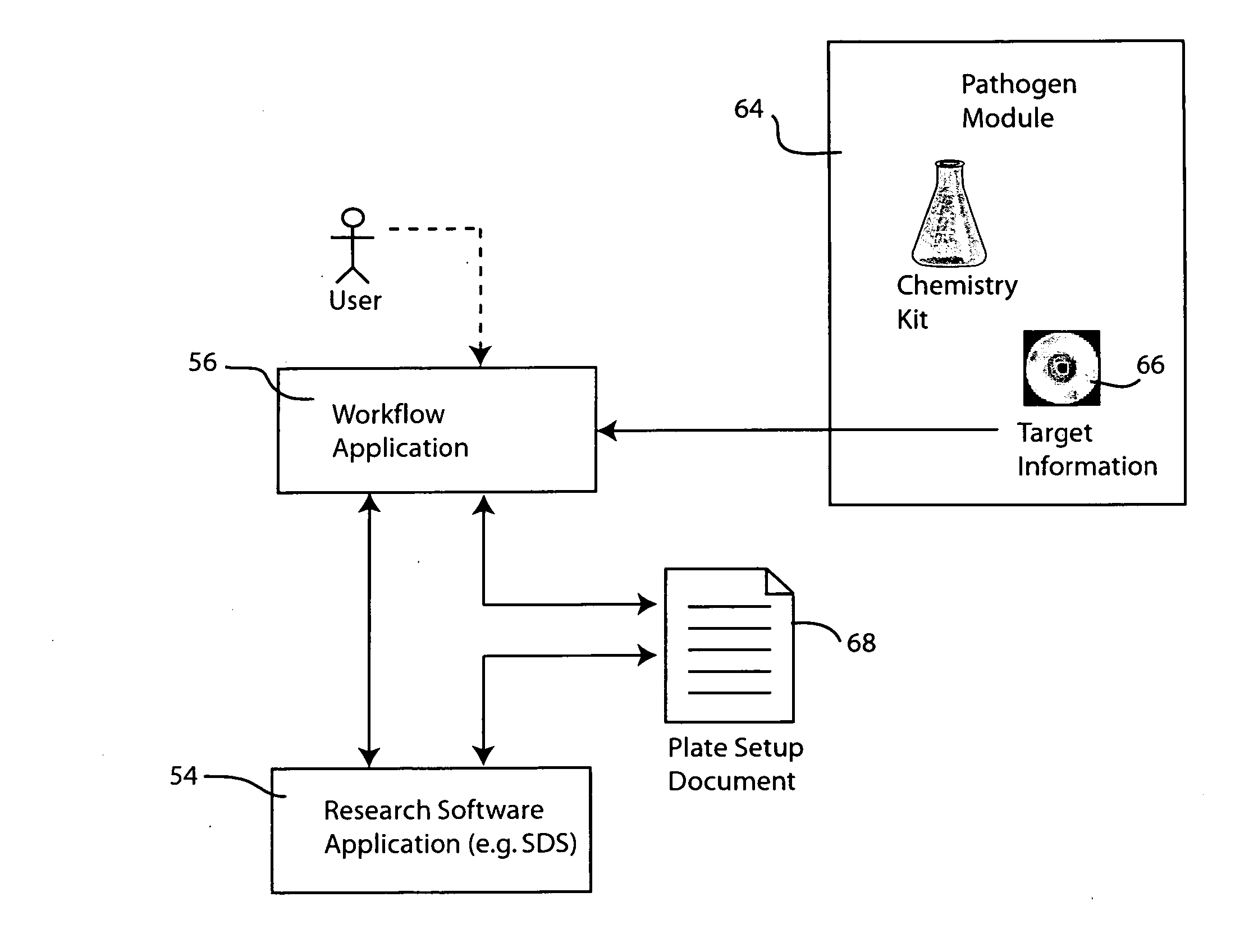
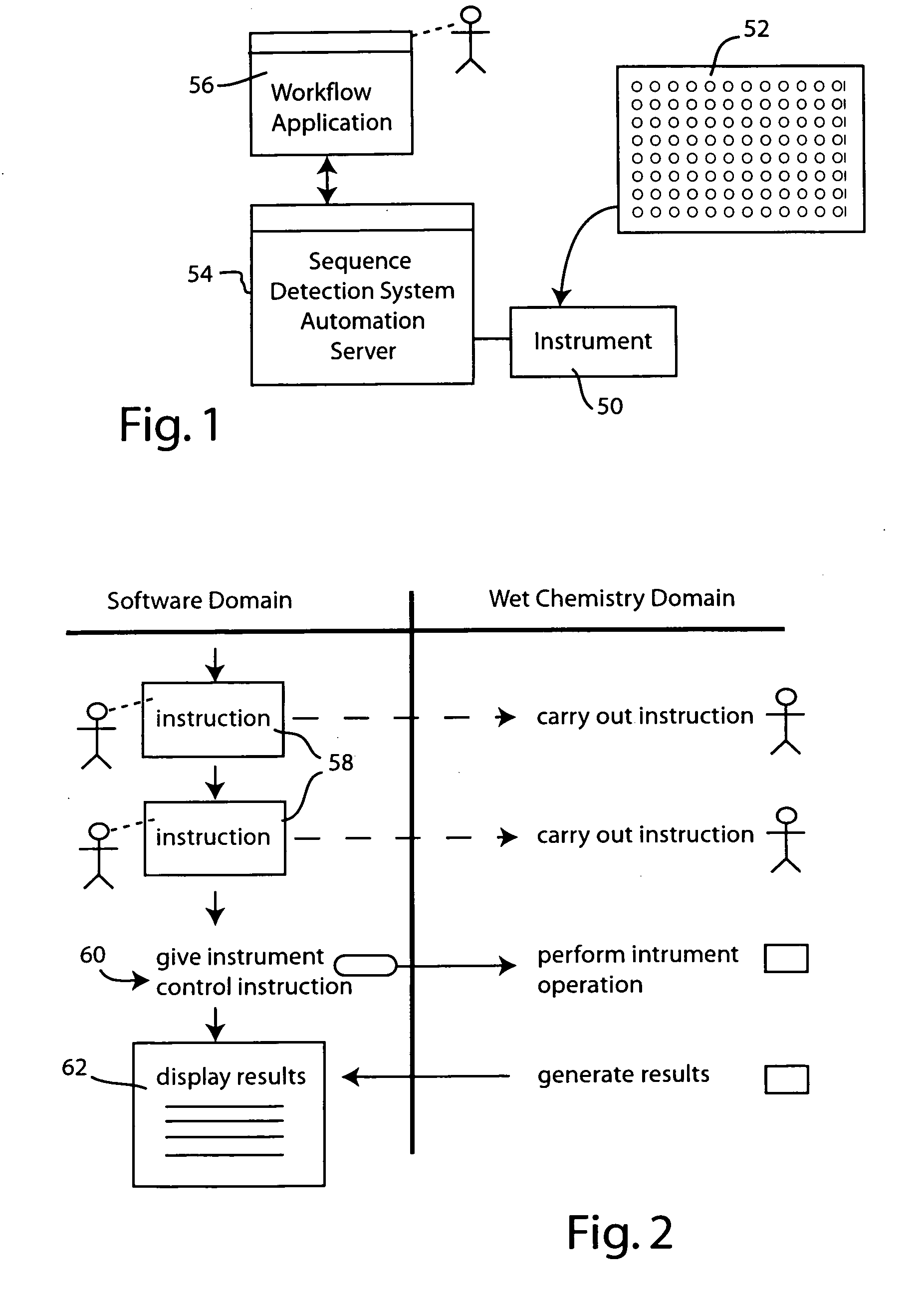
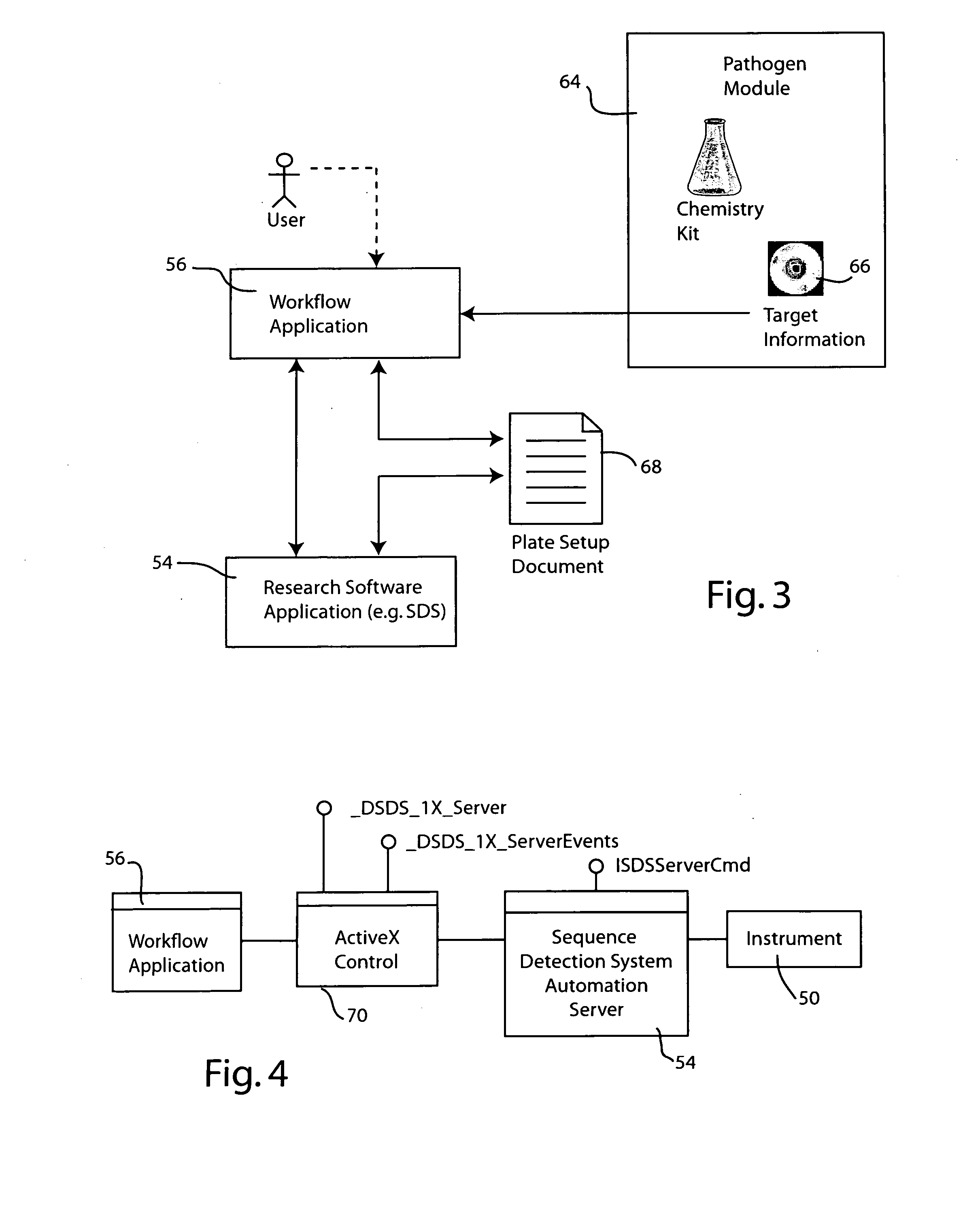
Systems and methods for generating automated software workflows for biological testing
InactiveUS20070112804A1Digital data processing detailsMicrobiological testing/measurementSoftware engineeringLearning curve
The workflow application integrates with a research software application associated with a laboratory instrument to provide a user with step-by-step instructions on how to follow the workflow steps of a laboratory experiment. The instructions are dynamically tailored, according to the nature of the workflow, the samples being experimented upon and / or the operating states of the instrument and / or the research software application. The workflow application significantly reduces the learning curve to operate sophisticated laboratory instruments. In a genetic testing instrument the workflow application can prescribe the need for control samples and can optimize the layout of samples within the instrument's sample receiving plate or fixture.
Owner:APPLERA
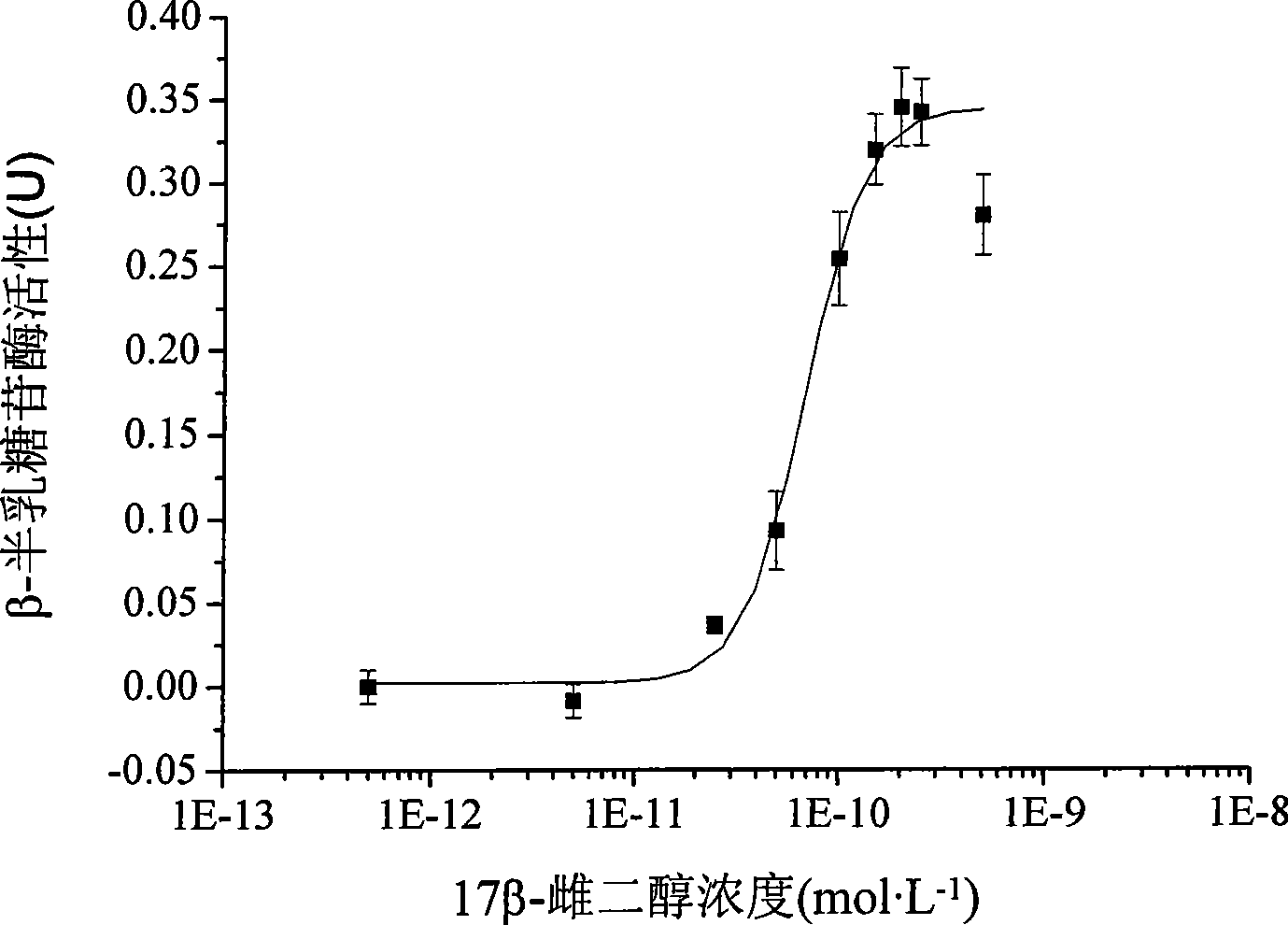
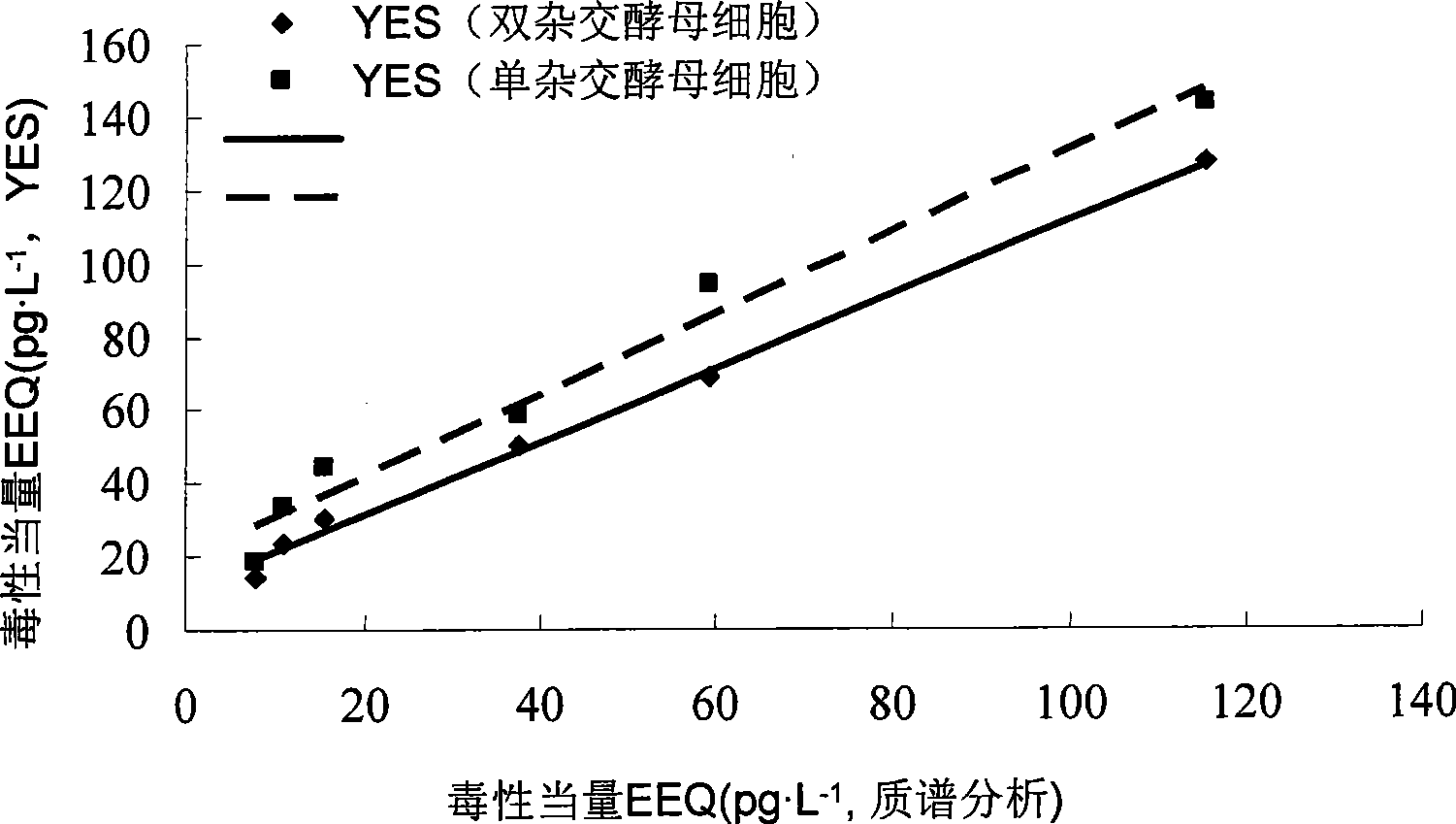
Two-hybrid yeast for detecting estrogen-like compound in environment and biological test method
ActiveCN101469315AThe test process is fastTest stableFungiMicrobiological testing/measurementBiotechnologyMammal
The invention provides a two-hybrid yeast for detecting estrogen-like compounds in environmental samples and a preparation method thereof, wherein the yeast contains pGBKT7-ER yeast expression plasmids and pGAD424-GRIP1 yeast expression plasmids, wherein the pGBKT7-ER yeast expression plasmids contain estrogen receptor genes, and the pGAD424-GRIP1 yeast expression plasmids contain estrogen receptor coactivated factor genetic fragments with the sequence of SEQ ID No.2. The invention also provides a bioassay method for detecting the estrogen-like compounds in the environment, which comprises: co-culturing two-hybrid yeast cells and a sample to be detected, adding a reaction liquid of o-nitrobenzene-beta-D-galactopyranoside for reaction, and calculating the concentration of the estrogen-like compounds according to the detected absorbance value of supernatant at 420 nanometers after the reaction stops. The invention adopts the two-hybrid yeast of recombinant estrogen receptor genes for test, and is more close to the actual action conditions of an endocrine system of a mammal; constructed yeast cell genes have stable character and are easy to culture and screen; the screening process of the whole estrogen-like effect is simple to operate; and the required quantity of the sample is small, and the cost is low.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Biological testing system
InactiveUS7641777B2Improved connectorImprove user experienceImmobilised enzymesBioreactor/fermenter combinationsContact padElectrical connection
Owner:ROCHE DIABETES CARE INC
Portable biological testing device and method
ActiveUS7910361B2Avoid biological contaminationBioreactor/fermenter combinationsBiological substance pretreatmentsAmbient pressureBiological Testing
A device and method for providing portable biological testing capabilities free from biological contamination from an environment outside the device are provided. The device includes a portable housing. The device further includes a volume surrounded by the housing and sealed against passage of biological materials between the volume and the environment outside the device. The device further includes a culture medium within the volume. The device further includes one or more ports configured to provide access to the volume while avoiding biological contamination of the volume. The device further includes a valve in fluidic communication with the volume and the environment. The valve has an open state in which the valve allows gas to flow from within the volume to the environment outside the device and a closed state in which the valve inhibits gas from flowing between the volume and the environment. The valve switches from the closed state to the open state in response to a pressure within the volume larger than a pressure of the environment outside the device.
Owner:BARNES ALLEN C +1
Biological testing system
InactiveUS20060052682A1Improved connectorImprove user experienceImmobilised enzymesBioreactor/fermenter combinationsContact padElectrical connection
A connector for establishing electrical connection between a testing device and a test strip with a biological fluid thereon includes a contact pad on the test strip, and one or more contact wires in the testing device. When the strip is inserted into the testing device, the end of the strip engages with a bight in the contact wire, pushing the contact wire in a direction normal to the direction of insertion. The movement of the contact wire forces a second portion of the wire against a part of the housing, thereby deforming the wire and moving another portion of the wire toward the contact pad. Some embodiments of the invention include 4, 6, 8, 15, or more contacts, which may be situated so as to receive the end of the test strip substantially simultaneously, or may be staggered in 2, 3, or more rows to spread out the resistance to movement presented.
Owner:ROCHE DIABETES CARE INC
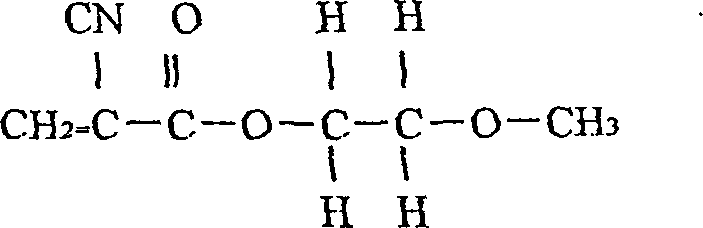
Biocompatible and photocurable polymers
ActiveUS9255922B2Microbiological testing/measurementMaterial analysis by electric/magnetic meansGlycidyl methacrylateMagnetic bead
The present invention relates to substrates for biological testing produced from photo-curable epoxy compositions which further include carboxyl-containing monomers such as acrylic acid, 2-carboxyethyl acrylic acid, 4-vinylbenzoic acid, or 3-Acrylamido-3-methyl-1-butanoic acid, or glycidyl methacrylate, etc. The photo-curable compositions may be used to cast films or fabricate beads, magnetic beads, or magnetic beads containing nickel barcodes. The resulting various kinds of films, beads, magnetic beads, or magnetic beads containing nickel barcodes may find use in clinical or biological applications.
Owner:APPLIED BIOCODE
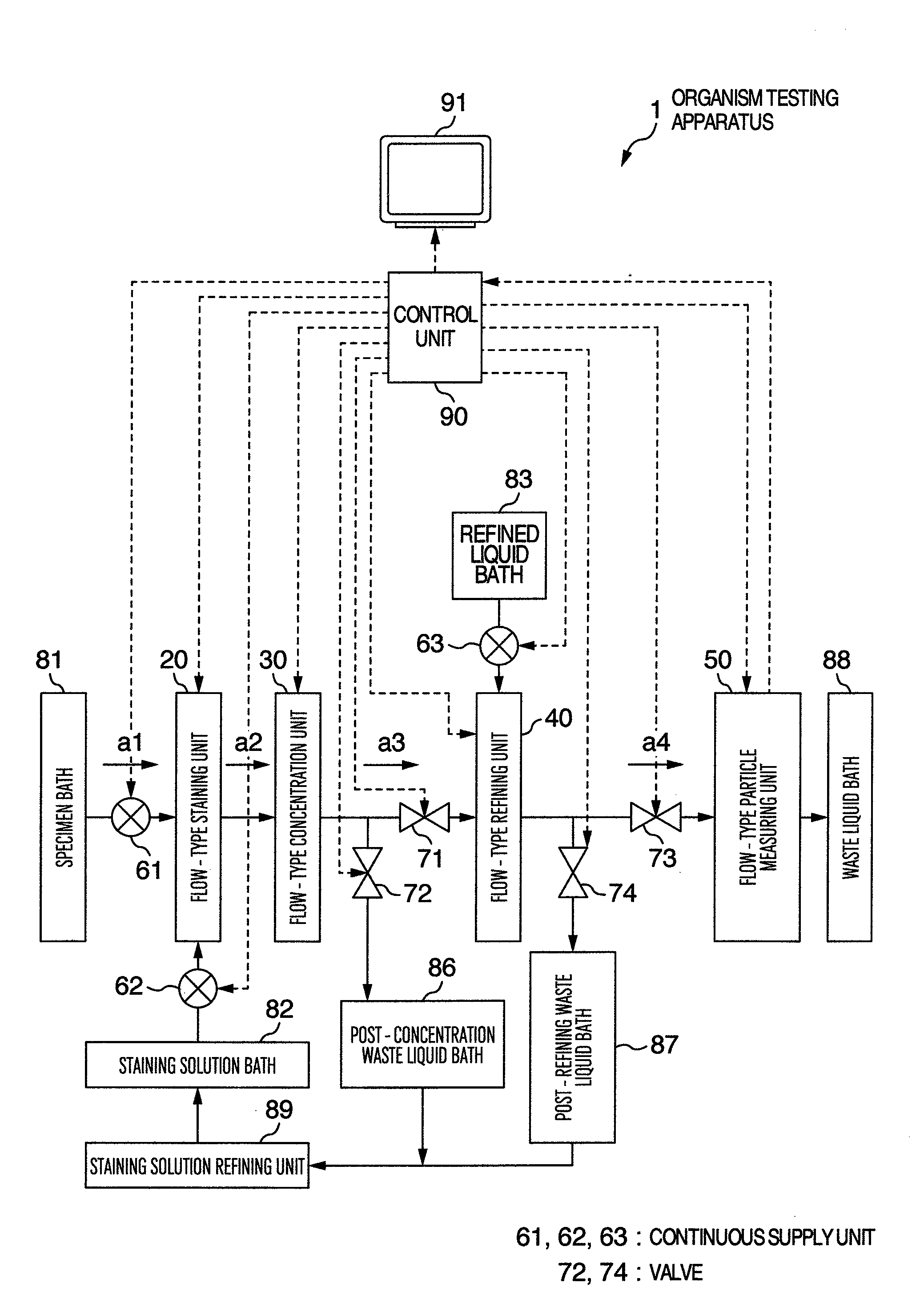
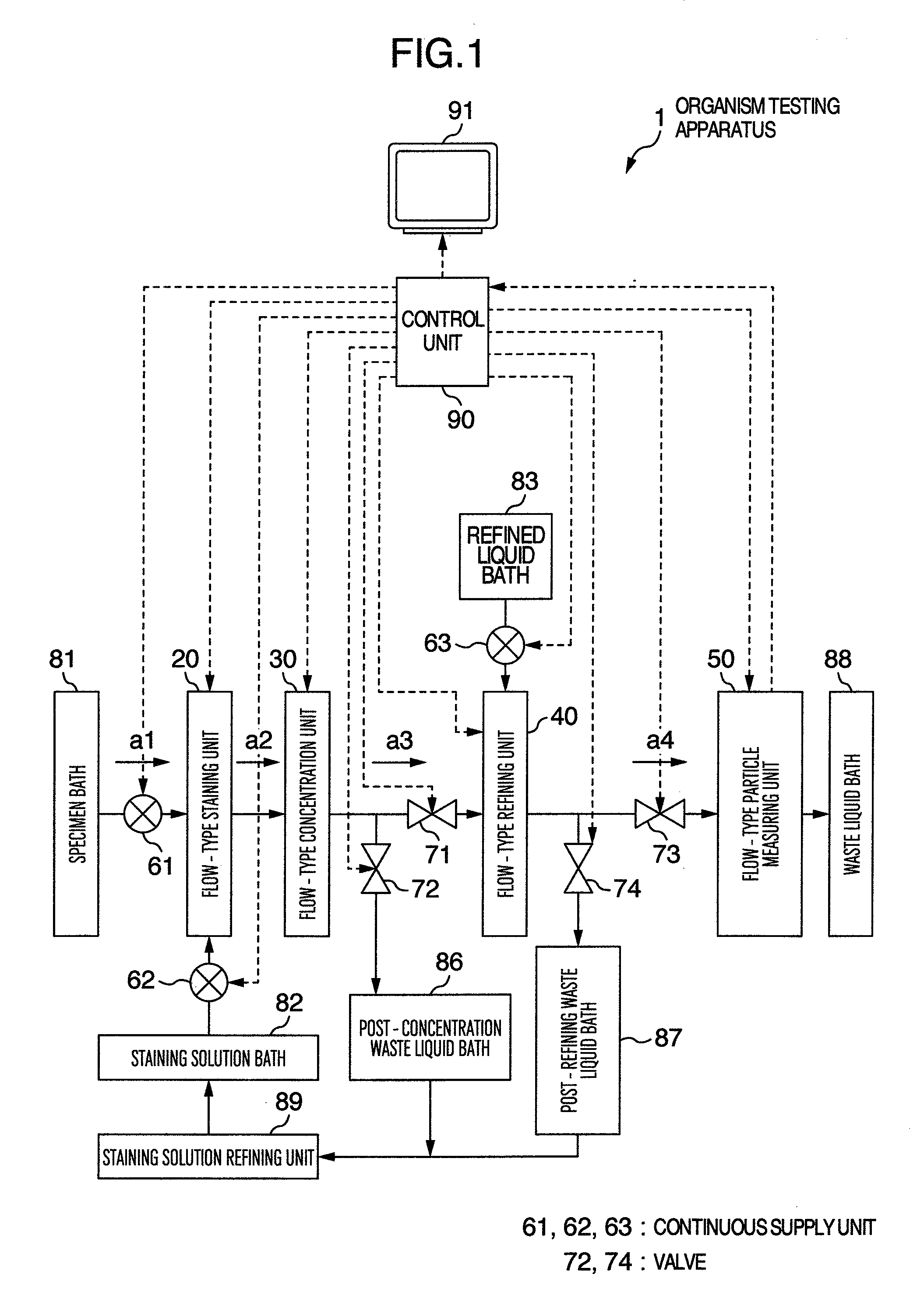
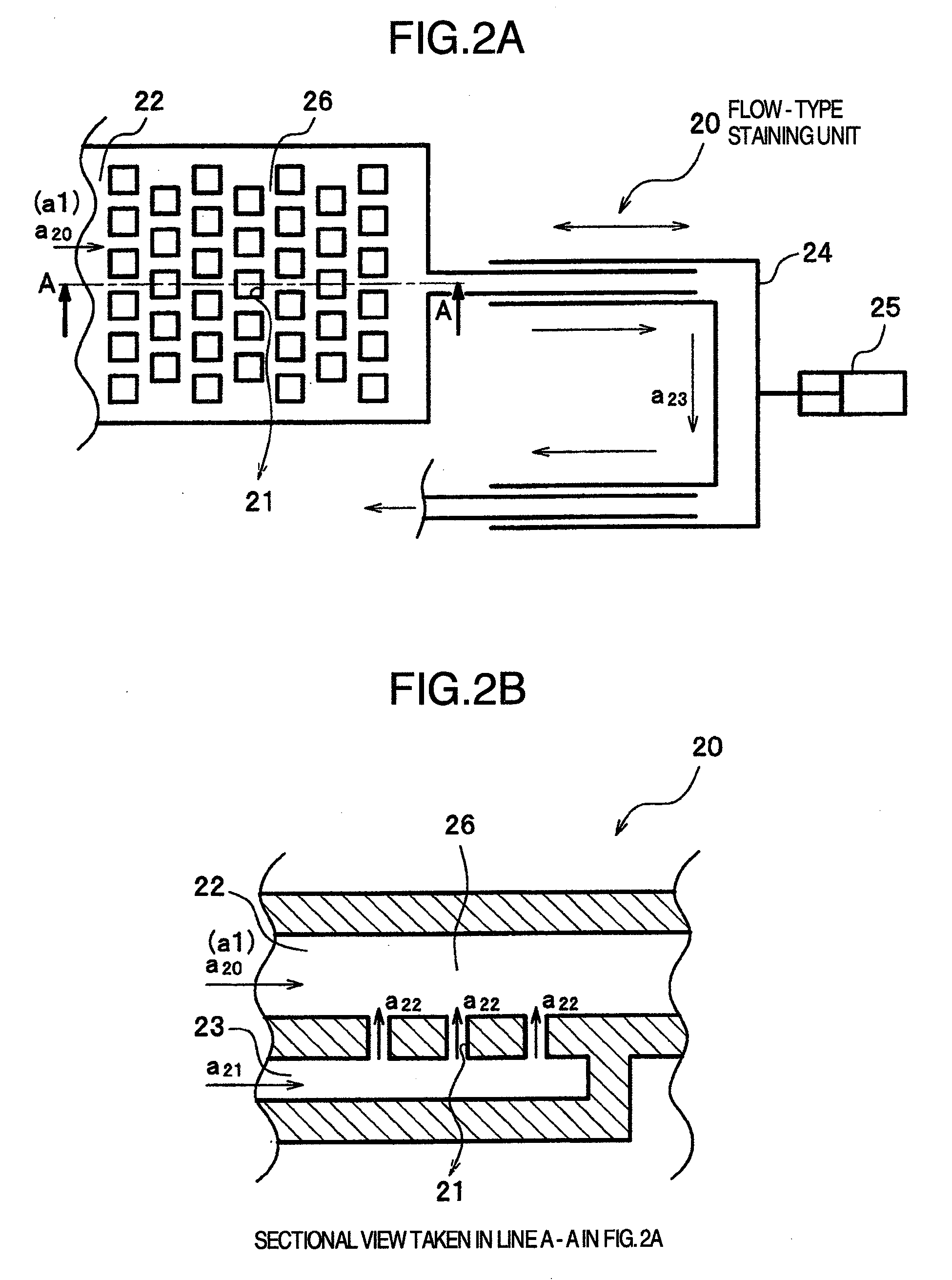
Organism testing apparatus
InactiveUS20090093045A1State of will deteriorateStable deathBioreactor/fermenter combinationsBiological substance pretreatmentsBiological bodyBiological Testing
An organism testing apparatus adapted to measure the organisms in a specimen in stable fashion is disclosed in which the organisms are stained, the specimen is concentrated and the information on the organisms contained in the specimen are acquired through a simple process. The apparatus includes a staining unit for staining the organisms having live cells existing in a flowing liquid specimen, a concentration unit for concentrating the organisms in a flowing stained specimen, an individual measuring unit for acquiring the image information on the individuals containing the organisms in the concentrated specimen, and a control unit for measuring the organisms based on the image information on the individuals output from the individual measuring unit.
Owner:HITACHI LTD
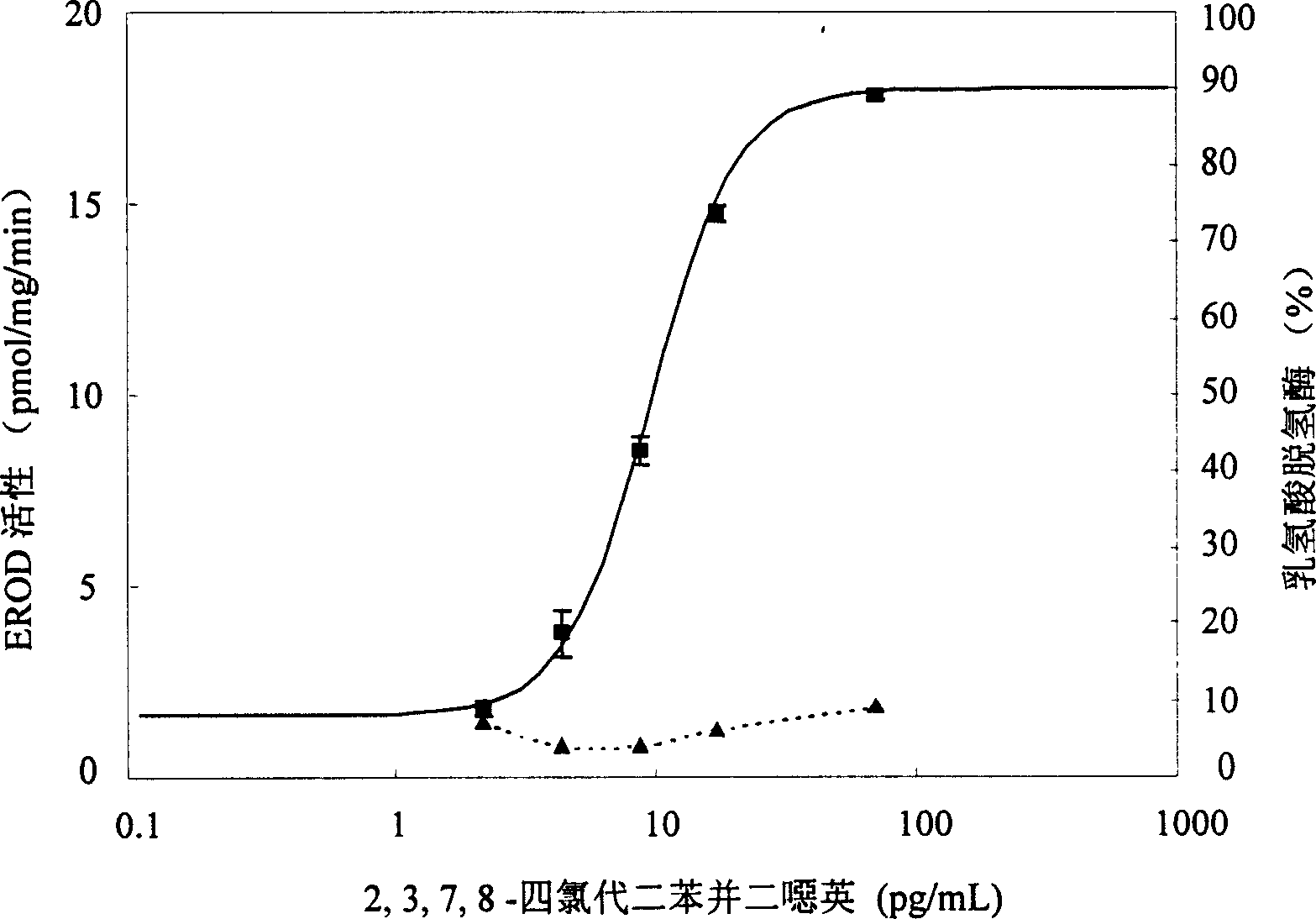
Biological measuring method for dioxins compound in monitoring environment samples
InactiveCN1548945AReduce the numberSave operating timeMicrobiological testing/measurementFluorescence/phosphorescenceDioxin CompoundBiological Testing
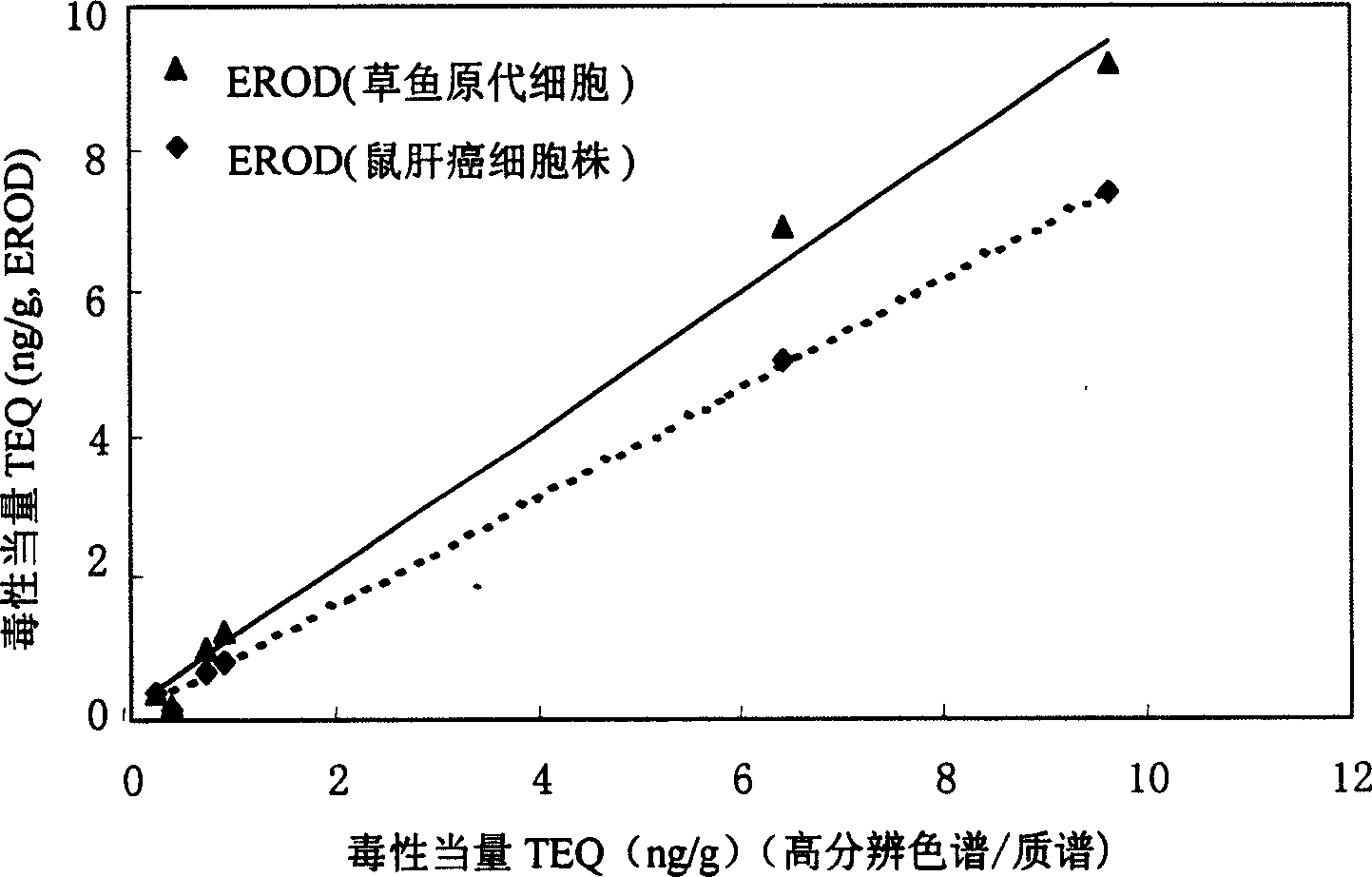
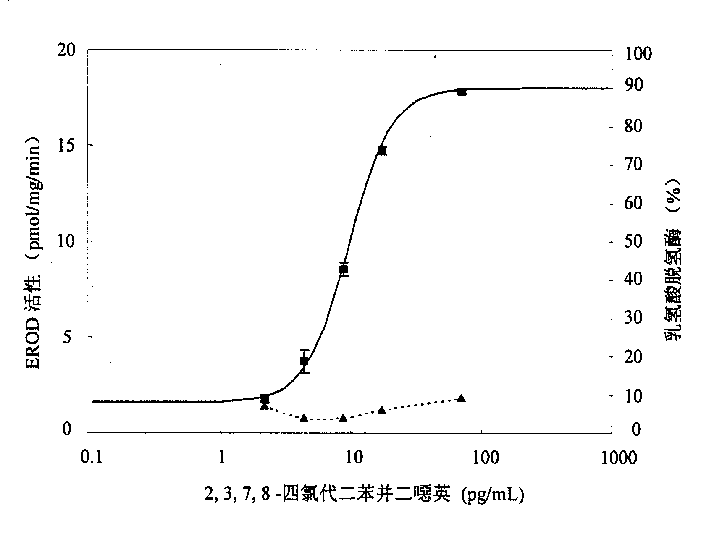
The present invention is the method of monitoring dioxins pollutant in environment sample with grass carp liver cell obtained via primary culture technology. Grass carp is blood eliminated via tail vein and its liver cell is primary cultured; the liver cell is then cultured together with 2, 3, 7, 8-tetrachlorodibenzyl dioxins; reactant solution containing excessive 7-oxethyl-isophenol azolactone is then added; the fluorescent strength of the end product 7-hydroxy-isophenol azolactone is measured with fluorophotometer and the fluorescent strength is used as parameter for expressing EROD enzyme activity to draw the standard curve of dosage of EROD enzyme activity of primary liver cell vs 2, 3, 7, 8-tetrachlorodibenzyl dioxins. The environment sample is detected similarly and through the comparison with standard curve the dioxins pollutant content in environment sample may be calculated.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
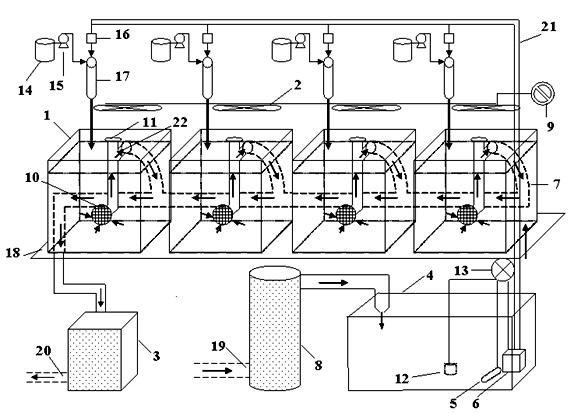
Online automatic dynamic exposure and water body treatment system in laboratory
InactiveCN102495188ARealize online processingIdeal for automatic dynamic exposurePreparing sample for investigationWater/sewage treatmentFully automaticPollution
The invention discloses an online automatic dynamic exposure and water body treatment system in a laboratory. The online automatic dynamic exposure and water body treatment system is characterized by comprising an online water flow system, a water body pretreatment system, an online chemical substance storage system, an online uniformly mixing system, an online polluted water body treatment system, an online temperature control system, an online illumination adjustment system and the like. According to a requirement for testing toxic organisms of water body pollutants in the laboratory, an online automatic dynamic exposure system in the laboratory is developed, so that a biologic test of toxic effects of the water body is realized; ideal laboratory dynamic exposure is realized through the automatic online temperature control system and the automatic illumination adjustment system; furthermore, exposed pollutants of the water body can be treated on line, and zero-pollution emission is realized. The system has a novel structure, a rational design and high practicability, and realizes fully automatic control.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
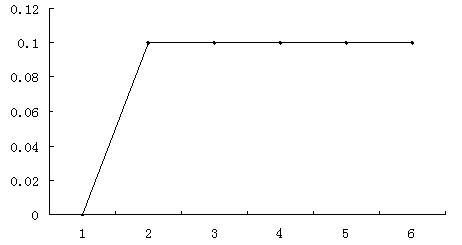
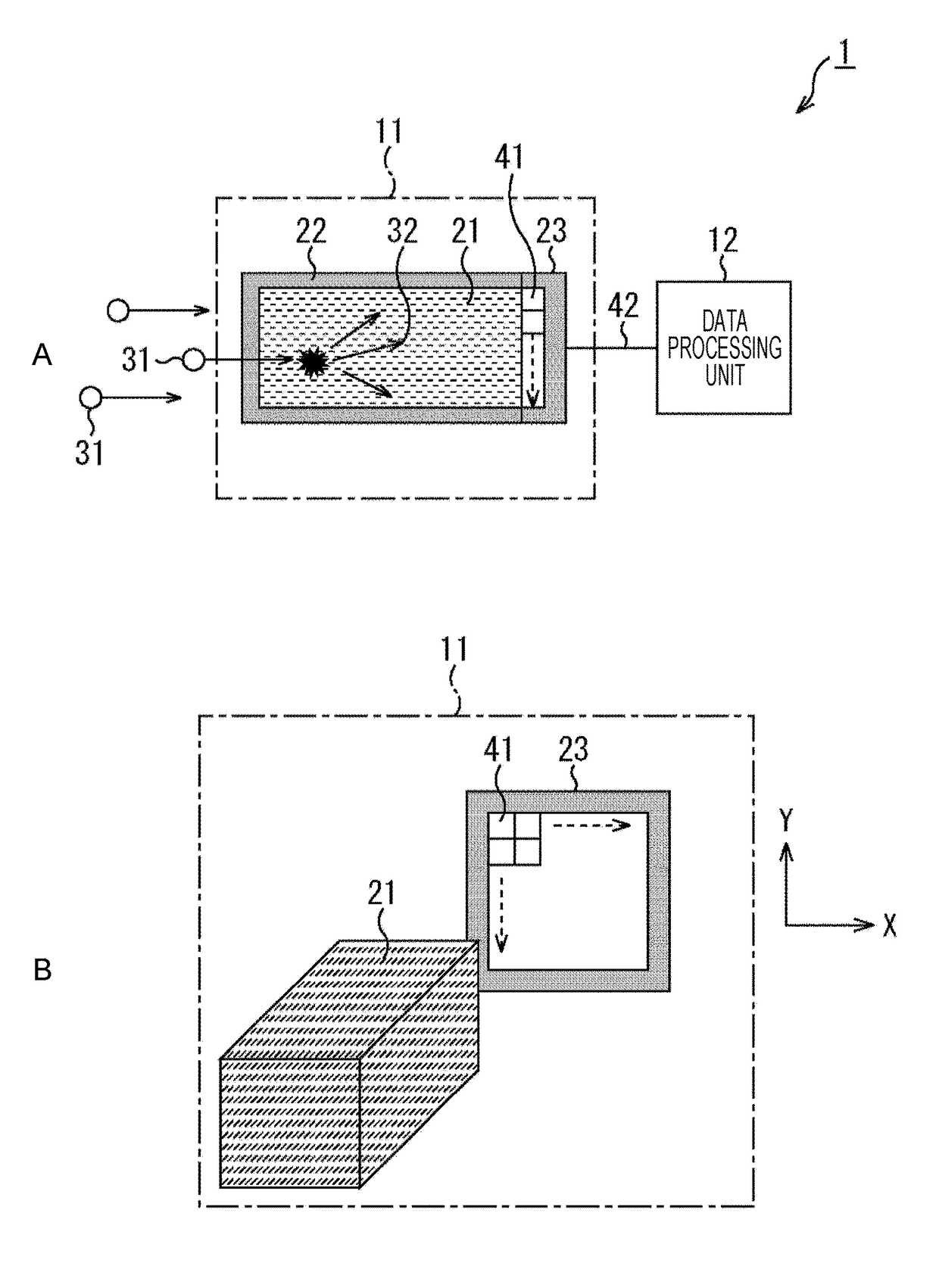
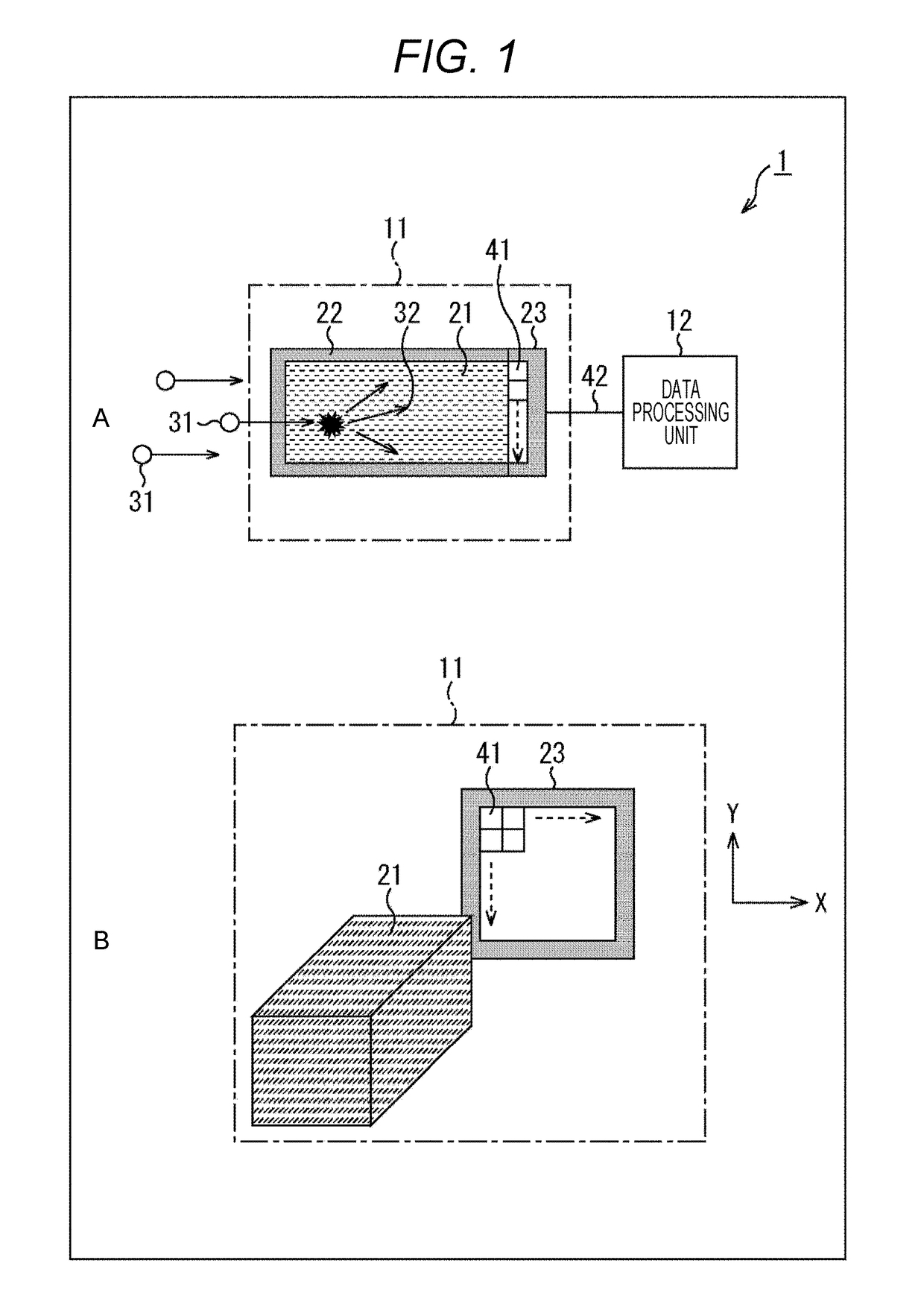
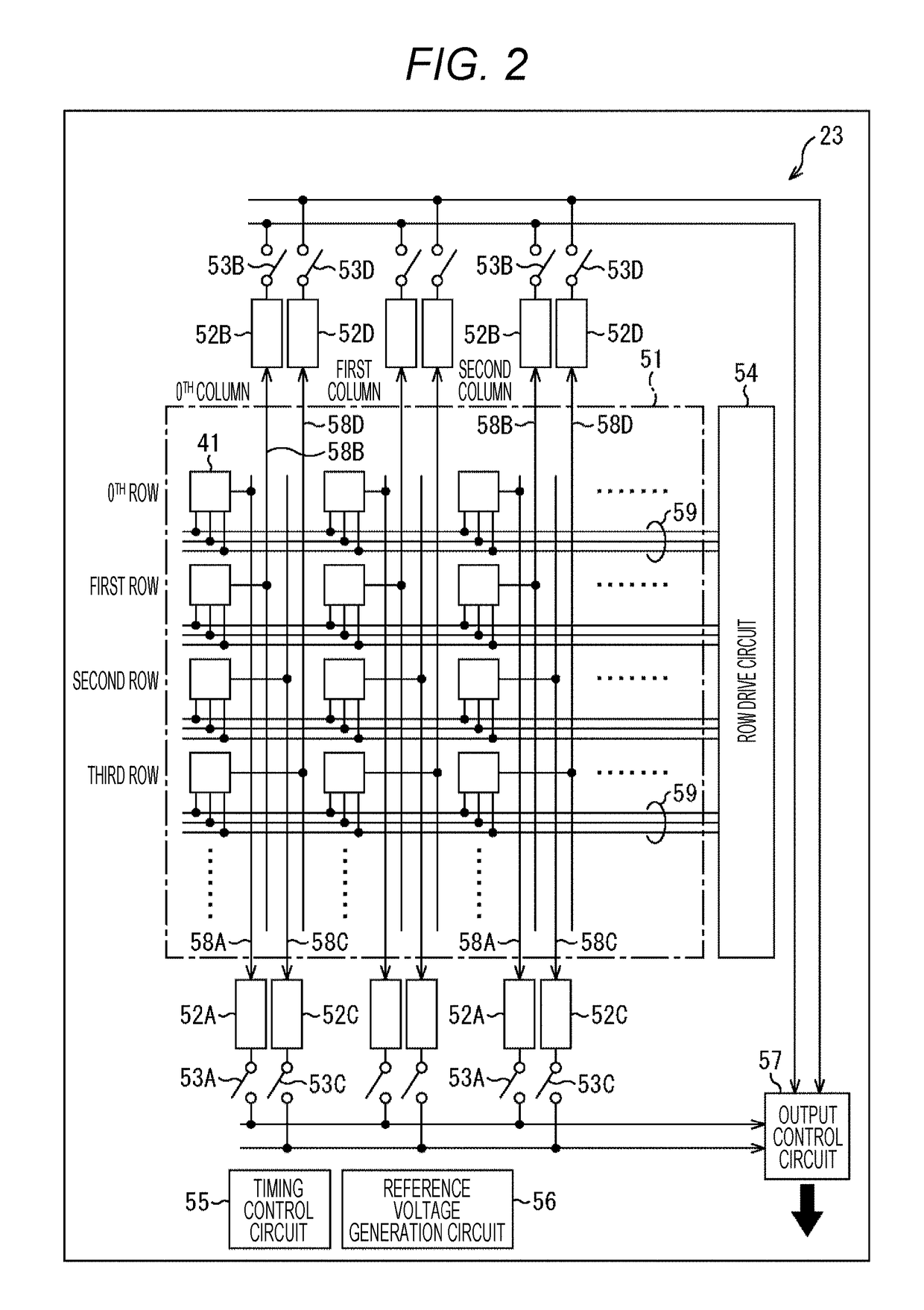
Optical pulse detection device, optical pulse detection method, radiation counter device, and biological testing device
ActiveUS20180328783A1Way accurateTelevision system detailsColor television detailsRadiation CounterBiological Testing
Owner:SONY SEMICON SOLUTIONS CORP
Culture medium its rpeparation method and method for culturing lact-streptocin
The present invention discloses a culture medium for producing lact-streptocin nisin its methods of preparation and method for culturing nisin. The preparation is put yeast protein, soy bean protein into jar, add water and mix, then put carbon, phosphate in turn, mix, debug PH6.2 - 7.2 into jar, add water, and high pressure steam, bactericide, cooling, connect food lactacide, breeding under the condiction of pressure is 0.1 - 0.5 MPa, temperature is 26 - 37 Deg. C for 24 - 72 hours, by biological testing the active of nisin is up to 4000 - 15000 IU / ml, then stop.
Owner:ZHEJIANG SILVER ELEPHANT BIO ENG
Biocidal blood glucose strip and lancet or sharps disposal device
A disposal receptacle having a biocidal interior surface for storing contaminated biotesting devices therein. The receptacle contains a one-way opening or valve to prevent used biotesting devices and bodily fluid from escaping or being removed from the receptacle; however, the one-way opening allows for insertion of the used biotesting devices therein. The biocidal interior surface and one-way valve in the receptacle form a sanitary method of containing contaminated biotesting devices. This combination allows the user to dispose of the receptacle, and safely and hygienically dispose of any contaminated biotesting devices and bodily fluid therein. The receptacle can be combined with a receptacle for unused biotesting devices, and / or sized to fit with or within a medical device, such as a test meter. In one form, the receptacle can be part of a care kit having a kit container for storing a lancing device, a testing device, and the disposal receptacle.
Owner:ROCHE DIABETES CARE INC
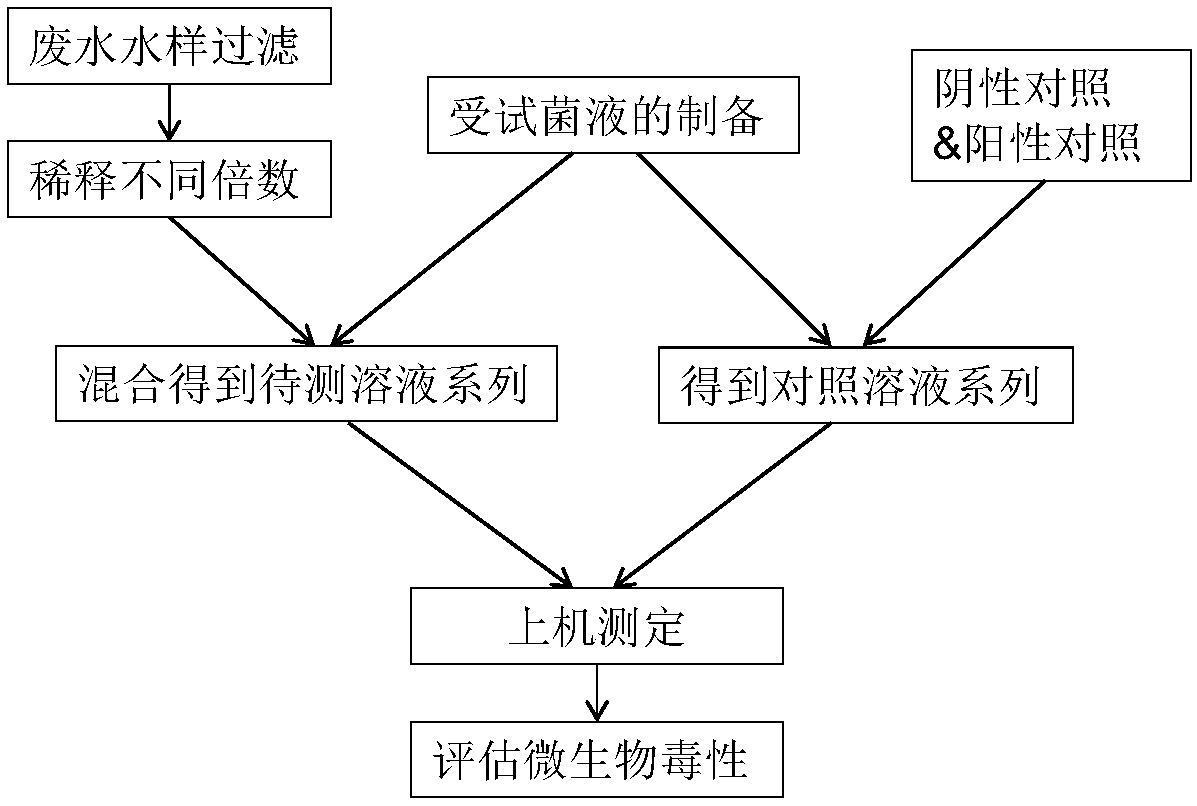
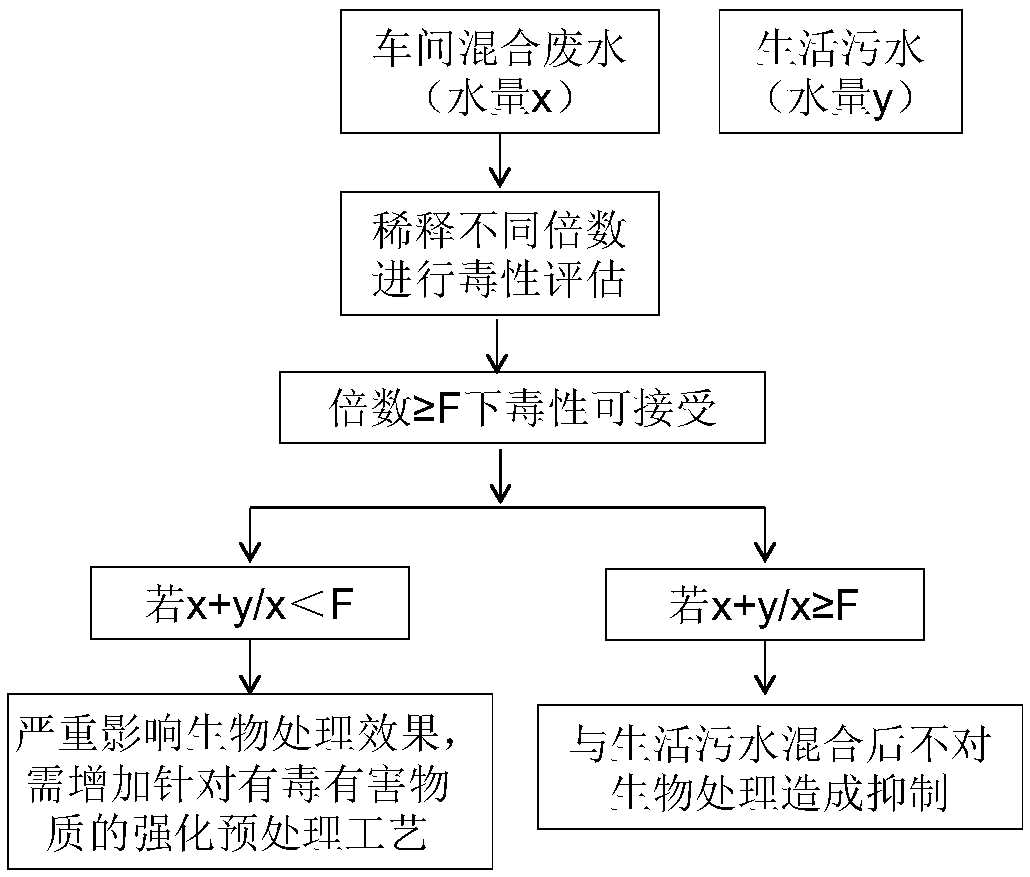
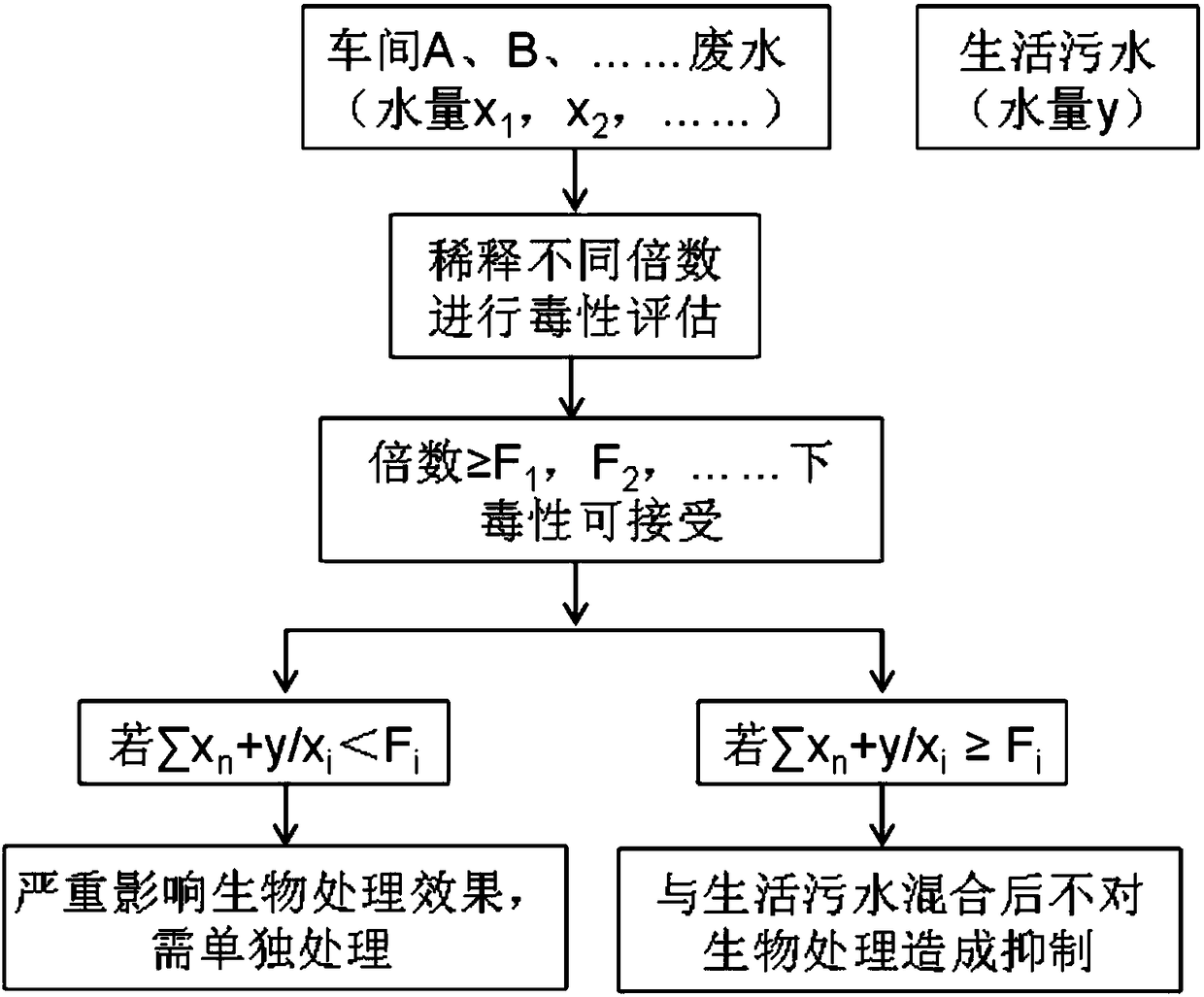
Method for evaluating toxicity of waste water on water treatment microorganisms
InactiveCN108459146AAssess overall toxicityHigh significance for practical applicationTesting waterMicroorganismWastewater
The invention discloses a method for evaluating toxicity of waste water on water treatment microorganisms. The method comprises the following steps that 1, a water treatment microorganism test bacteria solution is prepared; 2, to-be-tested waste water is diluted into multiple parts, and dilution ratios of the parts are different; 3, diluted waste water is added in the prepared water treatment microorganism test bacteria solution to be mixed to be uniform; 4, the amount of the microorganisms in each part of the mixed liquid obtained after uniform mixing is measured regularly or in real time inpreset time so as to obtain a microorganism growth curve of each part of mixed liquid; 5, the measured microorganism growth curve is compared with a blank control. The method has universality, biotoxicity of waste water of different types can be evaluated, and by means of the detection results of the biotoxicity of the waste water with different sources and dilution ratios, waste water separate treatment and (enhanced) pretreatment can be guided.
Owner:HUATIAN ENG & TECH CORP MCC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com