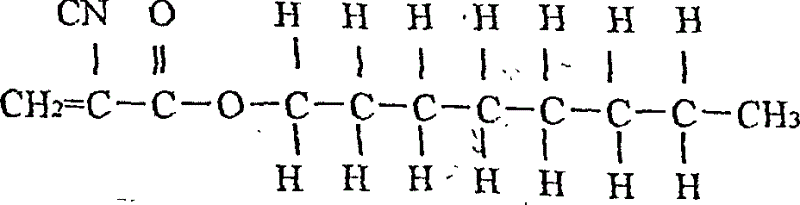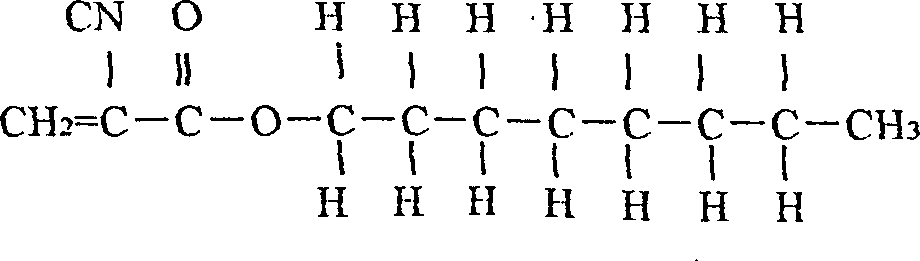Stopping bleeding spraying adhesive, preparing method and use thereof
An adhesive and compound technology, applied in surgical adhesives, applications, medical science and other directions, can solve the problems of low adhesive bonding strength, hepatitis C virus infection, anti-viral infection and other problems, achieve high bonding strength, prevent bacterial infection , the effect of increasing the bond strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The specific preparation method is as follows:
[0039] 1. Use raw materials with the following qualities:
[0040] (1). The monomers are 20 grams of n-octyl α-cyanoacrylate; and 80 grams of n-butyl α-cyanoacrylate; the content of both monomers is higher than 99%;
[0041] (2). Monomer appearance: colorless or light yellow transparent liquid;
[0042] (3). Monomer viscosity: lower than 3SPS;
[0043] (4). Monomer polymerization speed: 0.5-8 seconds;
[0044] Second, with glue
[0045] In a 100,000-level cleanliness workshop with constant temperature and humidity, the proportions of the monomers that are all qualified above are weighed in the above table: 20 grams of n-octyl cyanoacrylate; n-butyl α-cyanoacrylate Esters 80 grams; And according to α-cyanoacrylate n-octyl and α-cyanoacrylate n-butyl ester are loaded in the container (for example into the there-necked flask with stirrer), then stirred for 50 minutes;
[0046] 3. Filtration
[0047] The product ...
Embodiment 2
[0049]
[0050] The specific preparation method is the same as in Example 1.
Embodiment 3
[0053] The specific preparation method is as follows:
[0054] 1. Raw materials with the following qualities:
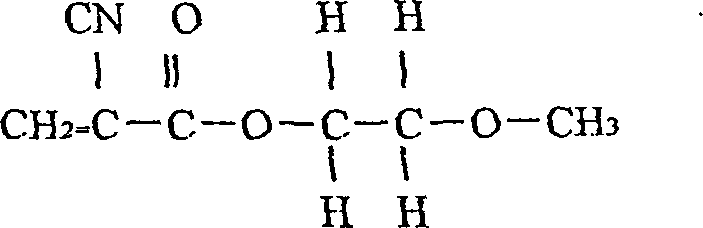
[0055] (1). Take the monomers as 30 g of n-octyl α-cyanoacrylate; 30 g of n-butyl α-cyanoacrylate; 40 g of methoxyethyl α-cyanoacrylate; The content is higher than 99%;
[0056] (2). Monomer appearance: colorless or light yellow transparent liquid;
[0057] (3). Monomer viscosity: lower than 3SPS;
[0058] (4). Monomer polymerization speed: 0.5-8 seconds;
[0059] Second, with glue
[0060] In the 100,000-level cleanliness workshop with constant temperature and humidity, the monomers that are qualified for the above indicators are weighed according to the proportions in the above table: 30 grams of n-octyl α-cyanoacrylate; Butyl 30 g; and methoxyethyl alpha-cyanoacrylate 40 g; Put into a container (for example, into a three-necked flask with a stirrer), then stir for 70 minutes;
[0061] 3. Filtration
[0062] The product obtained by stirring in the seco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com