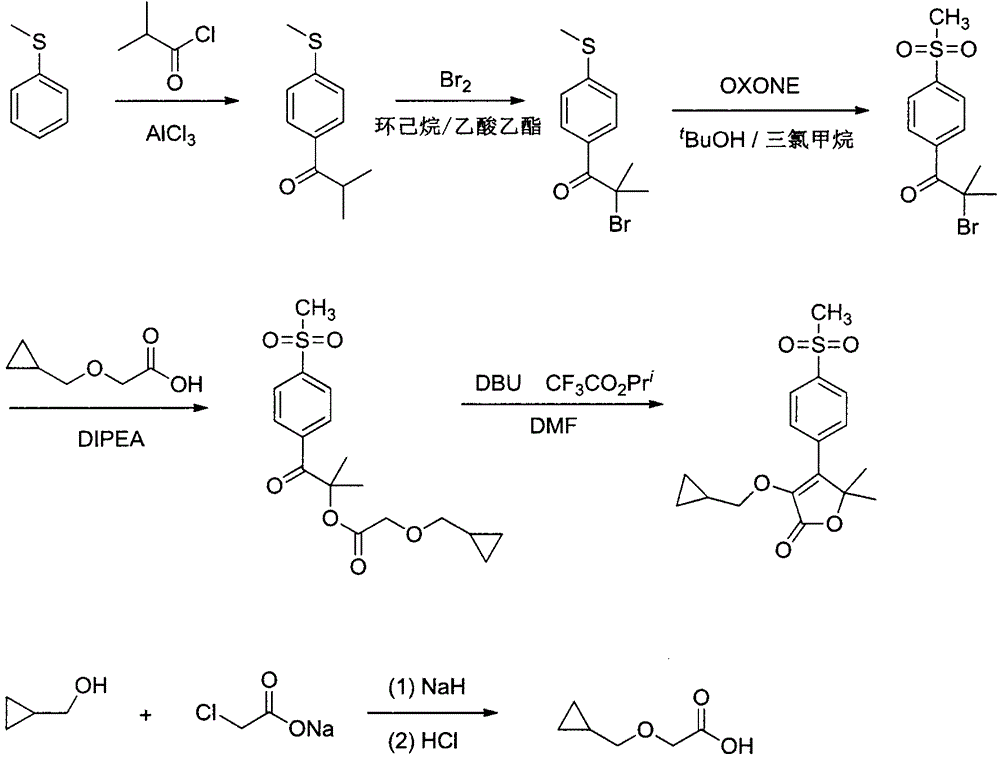
Firocoxib preparation method
A technology for felocoxib and acetone, which is applied in the field of synthesizing filocoxib, can solve the problems of poor repeatability and low yield of key steps, and achieve the effects of simple post-processing process, low cost and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] (1) Preparation of 2-methyl-1-(4-methylthiophenyl)-1-propanone
[0011] AlCl 3 (2.0g) was dissolved in chloroform (12mL), stirred and cooled to -10°C, wasobutyryl chloride (1.7mL) was added dropwise, stirred to dissolve, sulfide anisole (1.24g) was added dropwise, and the reaction was completed for about 1.5 hours. TLC monitoring, the reaction ended. It was quenched with water and the organic phase was separated. The organic phase was sequentially washed with saturated NaHCO 3 aqueous solution, saturated NaCl aqueous solution, anhydrous NaCl 2 SO 4 Dried, filtered, and spin-dried to obtain a colorless liquid, which was poured into a crystallization dish and evaporated to dry naturally to obtain a white solid with a yield of 99%. 1 H-NMR (500MHz, CDCl 3 )δ8.11(d, J=8.1Hz, 2H), 7.24(d, J=8.1, 2H), 3.49(m, 1H), 2.55(s, 3H), 1.18(d, 6H).
[0012] (2) Preparation of 2-methyl-1-(4-methylthiophenyl)-2-bromo-1-propanone
[0013] The product from the previous step (0.479...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com