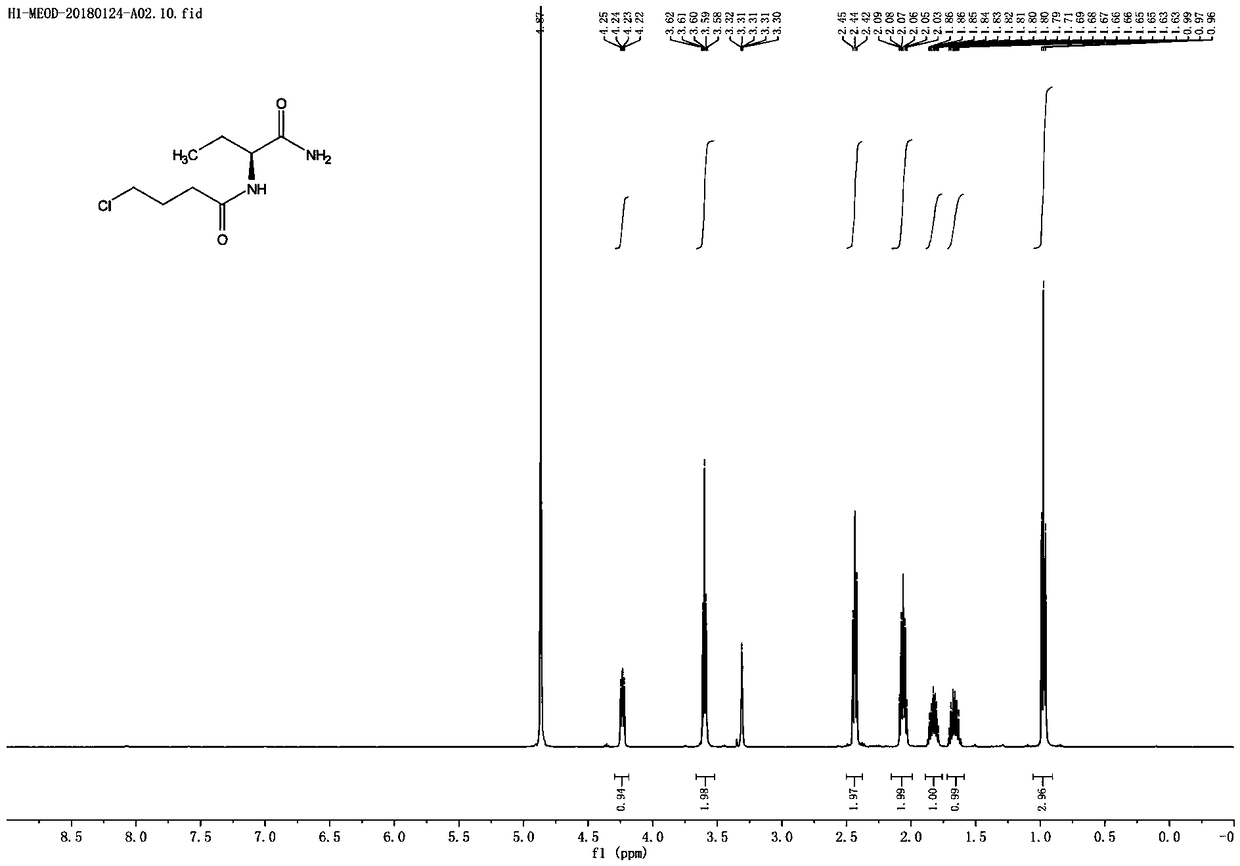
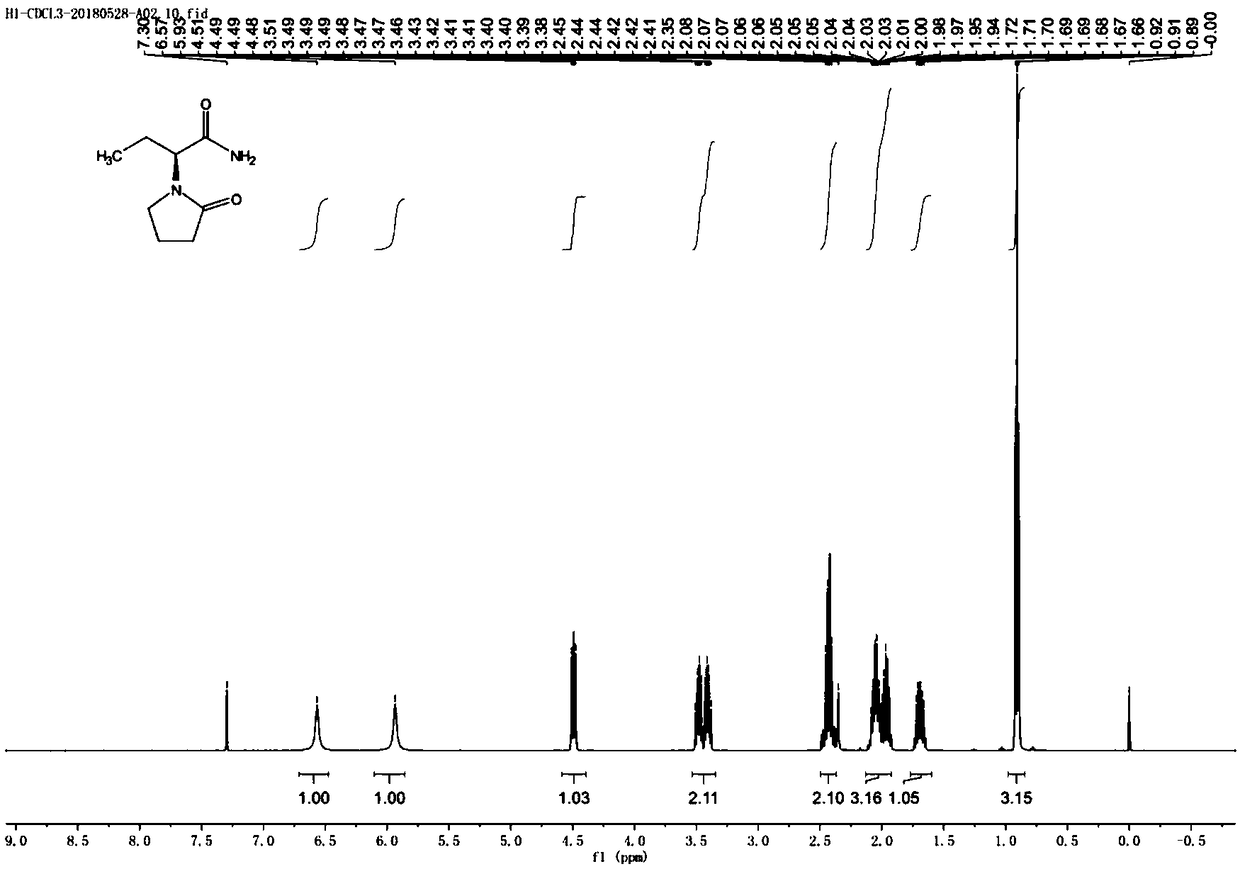
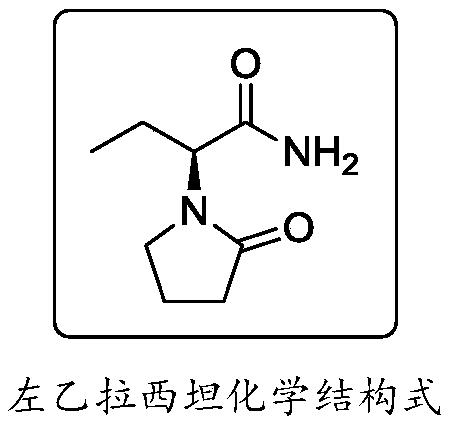
Synthesis process of levetiracetam
A technology of synthesis process and synthesis route, applied in the direction of organic chemistry, etc., can solve the problems of high cost input of hidden safety hazards and high optical isomers, and achieve the effect of low cost of safety input, high quality of finished products, and high environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The first step: the synthesis of (S)-2-(4-chlorobutanamide) butanamide
[0033] In a 2L reaction flask, add 1000mL of acetonitrile, add (S)-2-(4-chlorobutanamide)butyric acid (100.0g, 481.6mmol) under stirring, stir to dissolve, add (Boc) 2 O (126.1g, 577.8mmol), control the temperature in the reaction system to 20-30°C, slowly add pyridine (26.7g, 337.6mmol) dropwise to the reaction system for 20 minutes, and stir for 30 minutes after the addition is complete; Add ammonium bicarbonate (45.7 g, 578.1 mmol), and stir at 20 to 30°C for 5 to 8 hours; TLC spotting detects that the reaction of the raw materials is complete (developing solvent: ethyl acetate: glacial acetic acid = 100:1), and filter Remove the insoluble matter in the system, and concentrate the filtrate to dryness under reduced pressure; add the concentrate to acetone, heat to 60-70°C to dissolve until all dissolved, cool to 10-20°C, stir and crystallize for 2-3 hours; filter, reduce Drying under pressure ga...
Embodiment 2
[0037] The first step: the synthesis of (S)-2-(4-chlorobutanamide) butanamide
[0038] In a 2L reaction flask, add 1000mL of acetonitrile, add (S)-2-(4-chlorobutanamide)butyric acid (100.0g, 481.6mmol) under stirring, stir to dissolve, add (Boc) 2 O (126.1g, 577.8mmol), control the temperature in the reaction system to 20-30°C, slowly add pyridine (26.7g, 337.6mmol) dropwise to the reaction system for 20 minutes, and stir for 30 minutes after the addition is complete; Add ammonium carbonate (37.0g, 385.3mmol), stir and react at 20-30°C for 5-8 hours; TLC spot plate detection of raw material reaction is complete (developing solvent: ethyl acetate: glacial acetic acid = 100:1), filter to remove For the insoluble matter in the system, the filtrate is concentrated to dryness under reduced pressure; the concentrate is added to acetone, heated to 60-70°C to dissolve until completely dissolved, cooled to 10-20°C, stirred and crystallized for 2-3 hours; filtered, reduced pressure Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com