Method for preparing levetiracetam
A technology for aminobutyramide and aminolysis reaction, which is applied in the field of drug preparation, can solve the problems of complex process, long steps, low yield and the like, and achieves the effects of high resolution yield, short reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
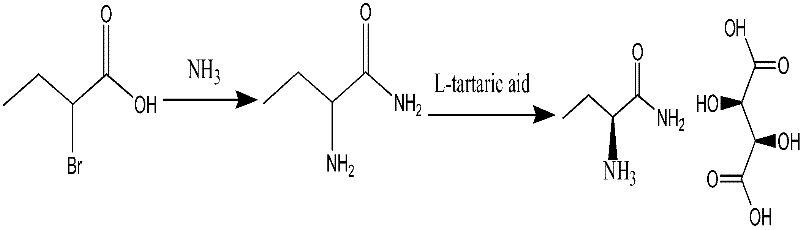
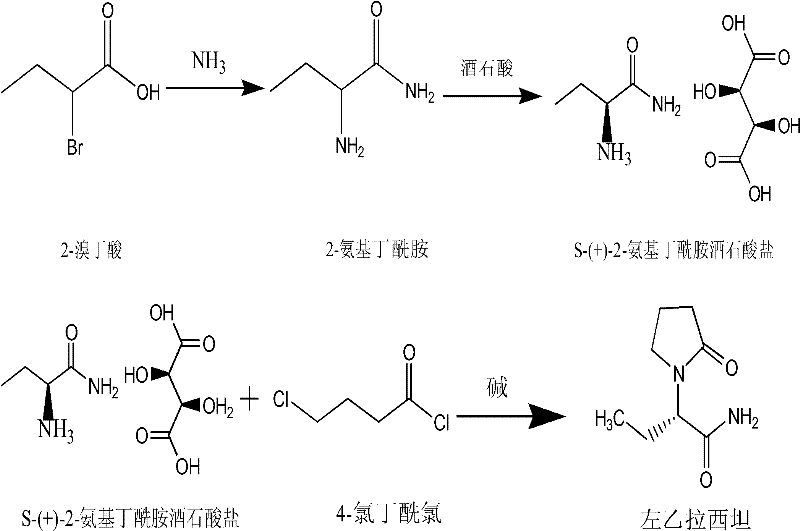
[0027] A preparation method of levetiracetam, comprising the steps of:
[0028] Step 1: 2-aminobutyramide synthetic method. React 500g of ammonia water and 60g of 2-bromobutyric acid at -5°C to 0°C for 3 to 5 days. After the reaction, concentrate to dryness, add 180ml of methanol to crystallize, and dry to obtain 33 grams of 2-aminobutyramide as a white solid. Molar yield ≥ 90%.
[0029] Step 2: the synthetic method of (S)-2-aminobutyramide tartaric acid
[0030] Dissolve 30 grams of 2-aminobutyramide in 200 ml of methanol, add 45 grams of L-(+)-tartaric acid, 0.3 grams of salicylaldehyde, heat up and reflux for 2 hours, cool and crystallize, and filter and dry the obtained S-(+)- 67 grams of 2-aminobutyramide tartaric acid, molar yield ≥ 90%
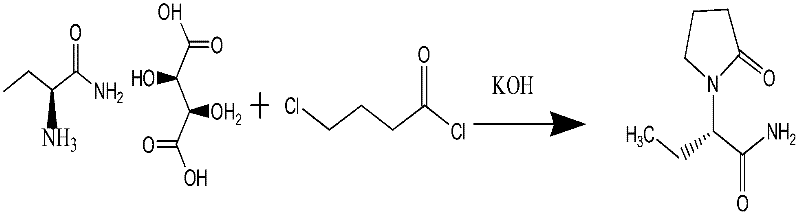
[0031] Step 3: the synthetic method of levetiracetam
[0032] 20 g of S-(+)-2-aminobutanamide tartaric acid was put into 120 ml of dichloromethane, 10 g of anhydrous sodium sulfate, 0.1 g of tetrabutylammonium bromide, and 25 g of p...
Embodiment 2
[0034] A preparation method of levetiracetam, comprising the steps of:
[0035] Step 1 is the same as Step 1 in Example 1.
[0036] Step 2: the synthetic method of (S)-2-aminobutyramide tartaric acid
[0037] Dissolve 30 grams of 2-aminobutyramide in 200 ml of methanol, add 45 grams of L-(+)-tartaric acid and 0.26 grams of benzaldehyde, heat up and reflux for 2 hours, cool and crystallize, and filter and dry the obtained S-(+)-2 - 67 grams of aminobutyramide tartaric acid, molar yield ≥ 90%
[0038] Step 3: the synthetic method of levetiracetam
[0039] 20 g of S-(+)-2-aminobutyramide tartaric acid was put into 120 ml of dimethyl tert-butyl ether, 10 g of anhydrous sodium sulfate, 0.1 g of tetrabutylammonium bromide, and 18 g of powdery sodium hydroxide were added. Control the temperature below 5°C, add 12g of 4-chlorobutyryl chloride dropwise, and keep warm at about 0°C for 5 hours after the drop is completed. After the reaction, filter, the filtrate is decompressed to re...
Embodiment 3
[0041] A preparation method of levetiracetam, comprising the steps of:
[0042] Step 1 and Step 2 are the same as Step 1 and Step 2 in Example 1.
[0043] Step 3: the synthetic method of levetiracetam
[0044] 20 g of S-(+)-2-aminobutyramide tartaric acid was put into 120 ml of acetonitrile, 10 g of anhydrous sodium sulfate, 0.1 g of tetrabutylammonium bromide, and 18 g of powdery sodium hydroxide were added. Control the temperature below 5°C, add 12g of 4-chlorobutyryl chloride dropwise, and keep warm at about 0°C for 5 hours after the drop is completed. After the reaction, filter, the filtrate is decompressed to recover the dichloromethane solvent, add acetone to the residue to recrystallize, and dry to obtain 12g of levetiracetam, the molar yield is ≥88%, the melting point is 116°C-120°C, and the purity is ≥99.9 %, single impurity ≤0.03%, total impurity ≤0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com