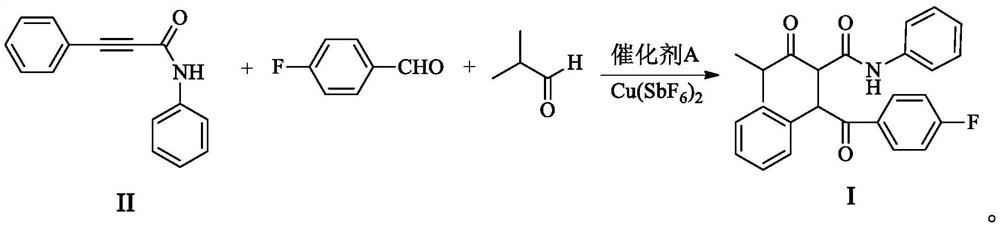
Method for synthesizing atorvastatin calcium intermediate by multi-component one-pot method
A kind of atorvastatin calcium, multi-component technology, applied in the one-pot synthesis of 4--2--3-phenyl-4-oxo-N-phenylbutanamide, multi-component one-pot method In the field of synthesizing atorvastatin calcium intermediates, it can solve the problems of high odor, high heat, irritation and corrosion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
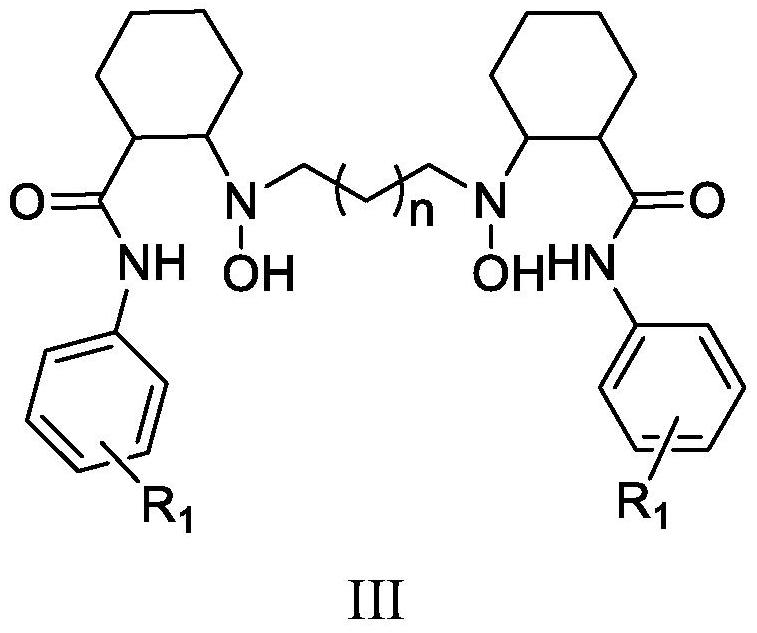
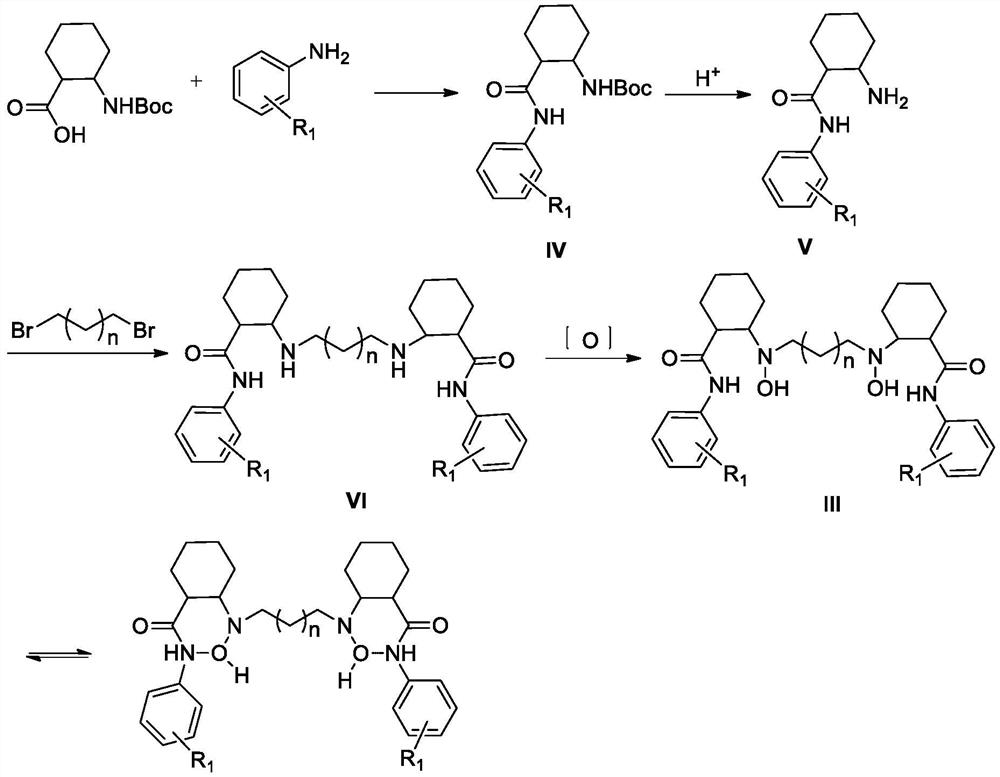
[0063] Embodiment 1: the preparation of formula III ligand
[0064] Preparation of Compound IV-1:
[0065]
[0066] 10 g of N-tert-butoxycarbonylaminocyclohexyl-2-carboxylic acid (41 mmol), 5.5 g of m-methoxyaniline (44.7 mmol, 1.08 eq) and 50 mL of dichloromethane were added to a 250 ml reaction flask. Stir and cool down to 0-10°C, then add 15.7g 1-propyl phosphoric acid cyclic anhydride (49mmol, 1.2eq), 1.5g 4-dimethylaminopyridine (12mmol, 0.3eq), 9.1g triethylamine (90mmol , 2.2eq). After the addition, keep the temperature at 0-10°C and stir the reaction for 8h. After the reaction, the reaction solution was concentrated to dryness under reduced pressure. Add 45mL of isopropanol to the concentrate and stir to make it clear, add dropwise 15mL of water, stir and crystallize at 0-10°C for 2h. After filtering, the filter cake was dried under reduced pressure at 35-45°C for 6-10 hours to obtain 13.6 g of compound IV-1. Yield 95.1%, HPLC purity 98.6%. ESI-MS: m / z 349.31[M+...
Embodiment 2
[0076] Embodiment 2: the preparation of formula III ligand
[0077] Preparation of Compound IV-2:
[0078]
[0079] 10 g of N-tert-butoxycarbonylaminocyclohexyl-2-carboxylic acid (41 mmol), 4.75 g of m-methylaniline (44.3 mmol, 1.08 eq) and 50 mL of dichloromethane were added to a 250 mL reaction flask. Stir and cool down to 0-10°C, add 17.3g 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (90mmol, 2.2eq), 1.5g 4-diimide hydrochloride to the reaction mixture Methylaminopyridine (12mmol, 0.3eq), 9.1g triethylamine (90mmol, 2.2eq). After the addition is complete, keep the temperature at 0-10°C and stir for 8 hours. After the reaction, the reaction solution was concentrated to dryness under reduced pressure. Add 45mL of isopropanol to the concentrate and stir to dissolve, add dropwise 15mL of water to the solution, and stir at 0-10°C for 2h. After filtering, the filter cake was dried under reduced pressure at 35-45°C for 6-10 hours to obtain 13.0 g of compound IV...
Embodiment 3
[0089] Embodiment 3: the preparation of formula III ligand
[0090] Preparation of compound IV-3:
[0091]
[0092] 10 g of N-tert-butoxycarbonylaminocyclohexyl-2-carboxylic acid (41 mmol), 6.05 g of m-dimethylaminoaniline (44.4 mmol, 1.08 eq) and 50 mL of dichloromethane were added to a 250 mL reaction flask. Stir the solution, cool the solution to 0-10°C, add 18.6g of dicyclohexylcarbodiimide (90mmol, 2.2eq), 1.5g of 4-dimethylaminopyridine (12mmol, 0.3eq), 9.1g of three Ethylamine (90mmol, 2.2eq). After the addition is complete, keep the temperature at 0-10°C and stir for 8 hours. After the reaction, the reaction solution was concentrated to dryness under reduced pressure. Add 45mL of isopropanol to the concentrate and stir to dissolve it, add dropwise 15mL of water to the solution, and stir and crystallize at 0-10°C for 2h. After filtration, the filter cake was dried under reduced pressure at 35-45°C for 6-10 hours to obtain 14.1 g of compound IV-3. Yield 95.3%, HP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com