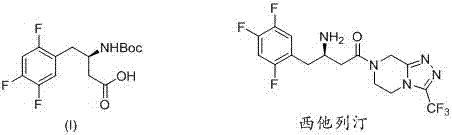
Method for synthetizing (R)-N-BOC-3-amino-4-(2,4,5-trifluorophenyl) butyric acid by adopting transaminase method
A technology of trifluorophenyl and transaminase, applied in the direction of fermentation, etc., can solve the problem of low total yield, achieve the effect of improving conversion rate, high yield, and simple and efficient synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
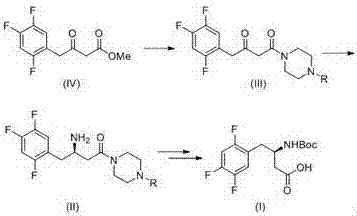
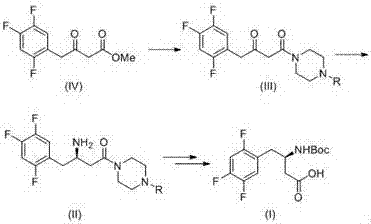
[0026] Example 1 : 4-(3- Oxo -4-(2,4,5- Trifluorophenyl ) Butyryl ) Piperazine -1- tert-butyl formate
[0027]
[0028] Dissolve 15.0 g of 3-oxo-4-(2,4,5-trifluorophenyl) methyl butyrate (1-1) and 14.0 g of N-Boc-piperazine in 100 mL of toluene, heat to reflux, and react After about 2 hours, TLC (PE / EA=3 / 1) monitored the complete reaction of the raw materials. The reaction solution was washed with water and salt water respectively, the organic phase was dried with anhydrous sodium sulfate, filtered, and spin-dried, and the obtained crude product was purified by column chromatography to obtain the product 4-(3-oxo-4-(2,4,5 -Trifluorophenyl)butyryl)piperazine-1-carboxylic acid tert-butyl ester (1-2) 24.3g, yield 99.7%.
Embodiment 2
[0029] Example 2 : ( R )-4-(3- Amino -4-(2,4,5- Trifluorophenyl ) Butyryl ) Piperazine -1- tert-butyl formate
[0030]
[0031] 50.0 g of tert-butyl 4-(3-oxo-4-(2,4,5-trifluorophenyl) butyryl) piperazine-1-carboxylate (1-2), 100 g of isopropylamine hydrochloride, Add 0.15 g of PLP to 500 mL of dipotassium hydrogen phosphate buffered saline system, adjust the pH to about 8.5 with a dilute solution of isopropylamine, and add 1.0 g of ω-transaminase freeze-dried powder. The reaction was controlled to pH=8.5 with 20% isopropylamine, converted at 45°C for 24 hours, and the reaction was completed by TLC monitoring. The solid was removed by filtration, the mother liquor was extracted 3 times with ethyl acetate, the combined organic phase was dried and concentrated to obtain a white solid (R)-4-(3-amino-4-(2,4,5-trifluorophenyl) Butyryl)piperazine-1-carboxylic acid tert-butyl ester (2-1) 43.1g, yield 85.7%, ee%=99.3%.
Embodiment 3
[0032] Example 3 : ( R )- N -BOC-3- Amino -4-(2,4,5- Trifluorophenyl ) butyric acid
[0033]
[0034] Dissolve (R)-4-(3-amino-4-(2,4,5-trifluorophenyl)butyryl)piperazine-1-carboxylic acid tert-butyl ester (2-1) 40.0g in 200mL methanol 50 mL of NaOH aqueous solution containing 10.0 g was added, heated to reflux for 5 hours, and the raw material disappeared on the TLC plate; then cooled to room temperature, 25.0 g of di-tert-butyl dicarbonate was added, and the reaction was continued at room temperature for 2 hours. Add 1 mol / L of potassium bisulfate to adjust the pH to be acidic, extract the product three times with dichloromethane from the reaction solution, dry and concentrate the combined organic phase, and crystallize the obtained crude product with petroleum ether to obtain (R)-N-BOC- 28.3 g of 3-amino-4-(2,4,5-trifluorophenyl)butanoic acid (3-1), yield: 85%, ee value: 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com