Preparation method of 3R-amino substituted butyrylamide derivative
A technology for butanamide and derivatives is applied in the field of preparation of 3R-amino-substituted butanamide derivatives, which can solve the problems of low production cost and high availability of chiral configuration, and achieves simple method, reduced waste liquid discharge, and reaction selection. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
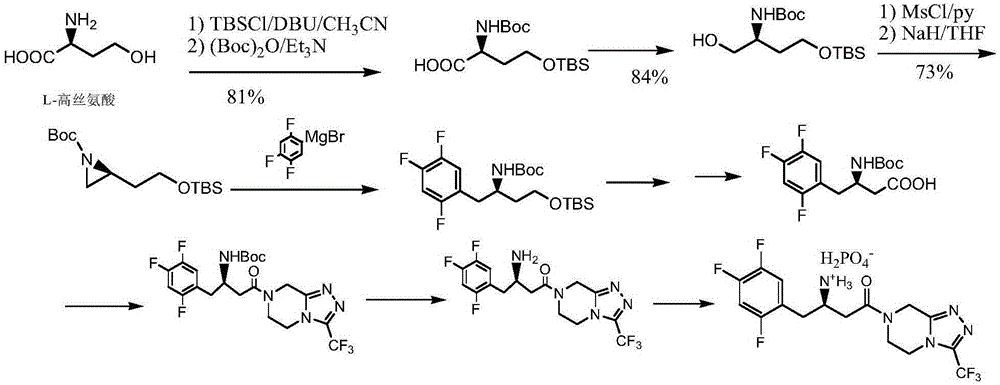
[0080] Embodiment 1: the preparation of compound Ia
[0081] Add 60 grams of isopropanol, 8.2 grams (20 millimoles) of enamine compound IIa, 3.5 grams (23 millimoles) of R (-) mandelic acid, 80 milligrams of 50% Raney nickel (water 50%), nitrogen replacement for 3 times, then stirred and reacted for 4 hours at an internal temperature of 20-25° C. and a hydrogen pressure of 1-2 atmospheres. The catalyst was recovered by filtration, the filtrate was cooled to 0-5° C., and filtered to obtain Ia. After drying, the single-pass yield was 35.6%. Gained filtrate is added in 100 milliliters flasks again, add 1.1 gram hydroperoxide isopropyl groups (its add-on calculates according to the amount of Ⅲ in the mother liquor and obtains, and calculation formula is m=hydrogen peroxide molecular weight*IIa millimole number*1.1* (1-one-way yield)), stirred and reacted at an internal temperature of 20-25° C. for 3 hours, and then heated to distill 5 grams of isopropanol. The mixture was transf...
Embodiment 2-9
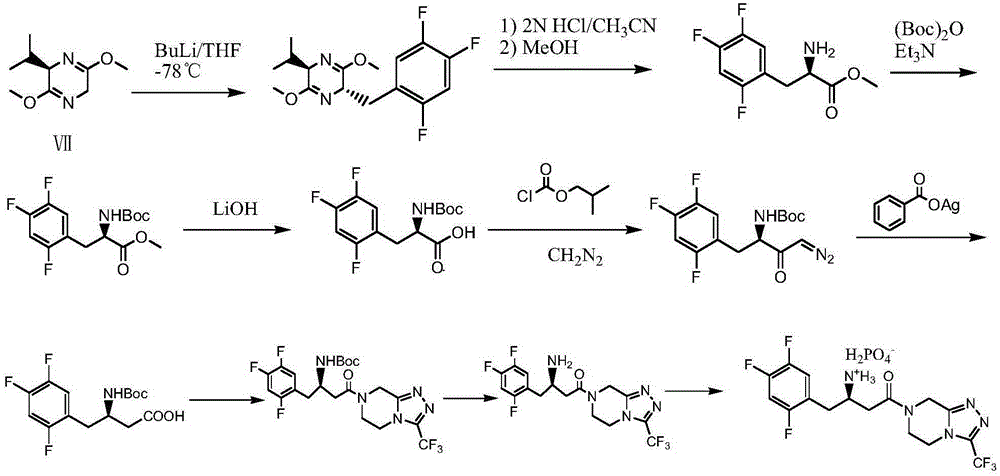
[0084] Example 2-9: Substituting IIb, IIc, IId, IIe, IIf, IIg, IIh, and IIi for IIa in Example 1 to prepare Ib, Ic, Id, Ie, If, Ig, Ih, and Ii compounds respectively, and feeding In terms of the amount of substance (number of moles) and IIa are the same, the method of operation is the same as in Example 1, and the single-pass yield and cumulative yield are listed as follows:
[0085] Table 2
[0086]
[0087]
Embodiment 10
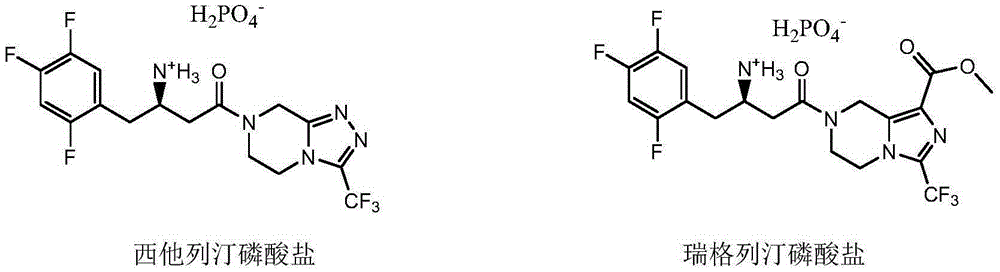
[0088] Embodiment 10: Preparation of sitagliptin phosphate
[0089] The product Ia of Example 1 was used to prepare sitagliptin phosphate through phosphoric acid salification. The specific operation of this step can be done according to the prior art, refer to Tetrahedron Letters2013, 54(50)6807.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com